Figures & data
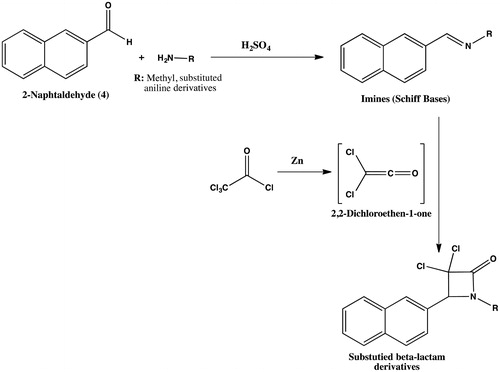
Table 1. The synthesis of imine derivatives (10–14).
Table 2. The addition reactions of imines and dichloroketene compounds.
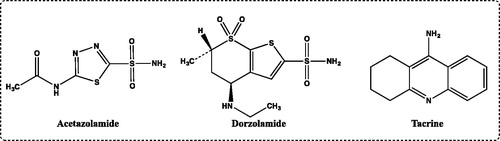
Figure 1. Standard compounds used for carbonic anhydrase I and II isoenzymes (acetazolamide and dorzolamide) and acetylcholinesterase (tacrine) inhibitors.