Figures & data
Figure 1. A placenta-on-a-chip microdevice: (A) The microengineered device is composed of the upper (blue) and lower (red) PDMS chambers separated by a vitrified collagen membrane. (B) Endothelial cells and trophoblasts are co-cultured in close apposition on the opposite sides of the intervening membrane to form a microengineered placental barrier in the placenta-on-a-chip device.
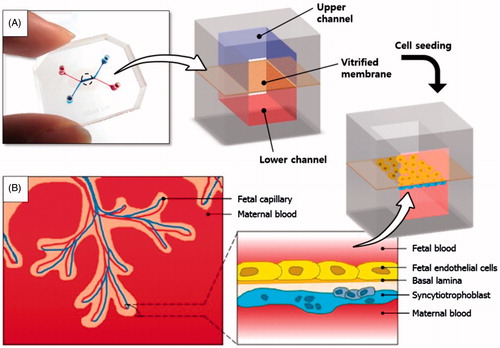
Figure 2. Fabrication of the placenta-on-a-chip: (A) Production of a microchannel master using photolithography. (B) Soft lithography-based replica molding of PDMS microchannels and the formation of the vitrified collagen membrane. The upper PDMS slab is permanently bonded to the lower slab containing a microchannel covered with the vitrified membrane. (C) Sequential seeding of JEG-3 cells and HUVECs in the multilayered microfluidic device.
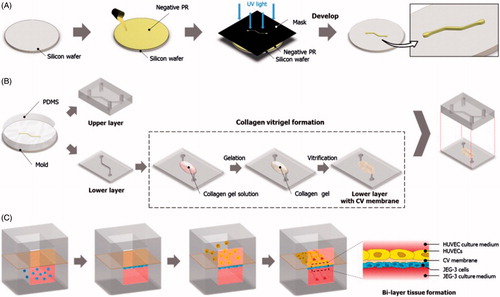
Figure 3. A microengineered placental barrier: fluorescence micrographs of (A) JEG-3 cells (red) and (B) HUVECs (green) grown on the lower and upper membrane surfaces, respectively. (C) A merge of A and B.
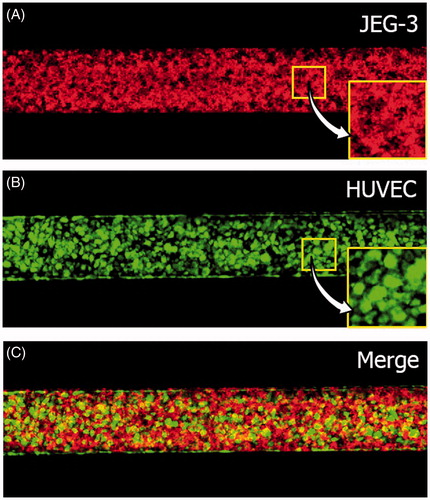
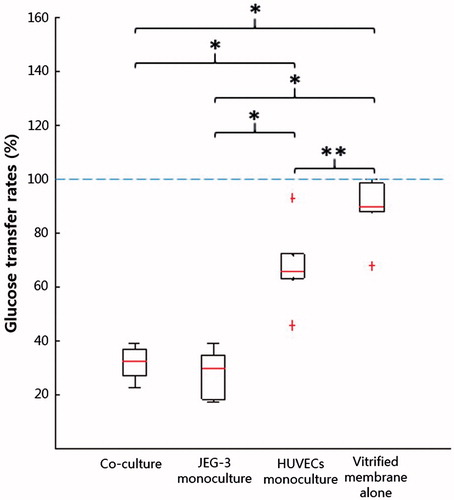
Figure 4. Changes in the amounts of glucose: (A) Glucose increases in the fetal compartment; (B) Reduction in glucose concentration in the maternal chamber. Boxes denote the interquartile range, with the red horizontal line representing the median. Whiskers extend from the box to the extreme values unless there were outliers (+ red signs).
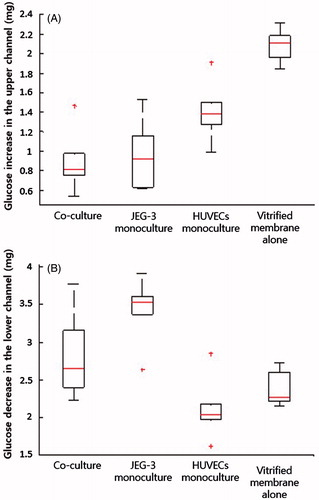
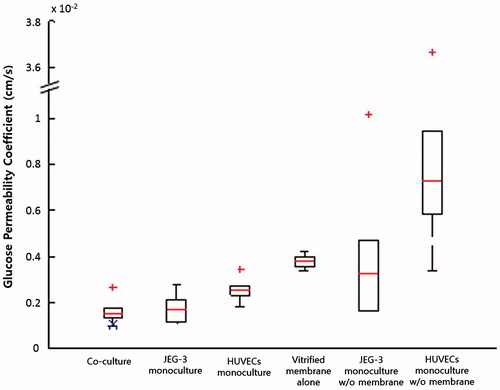
Figure 6. Glucose permeability coefficients. GPBL,derived calculated using Equation (Equation4(4) ) is marked with a blue X in the co-culture group.