Figures & data

Figure 2. Probability of treatment discontinuation in roflumilast and placebo groups in trials M2–124 (A) and M2–125 (B). Reproduced from Calverley PMA et al Lancet 2009;374:685–94 with permission. *Number of patients still at risk at the beginning of the respective week; number at risk might be different from the number completing the study because the protocol allowed patients to finish the study up to 7 days before the end of week 52.
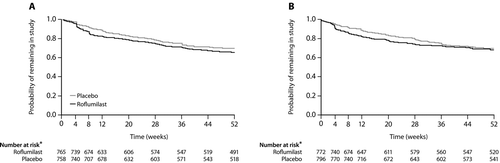
Figure 3. Prebronchodilator and postbronchodilator forced expiratory volumes in 1 s (FEV1) over 52 weeks in patients in roflumilast and placebo groups in trials M2–124 (A) and M2–125 (B), and changes in prebronchodilator and postbronchodilator FEV1 over 52 weeks in patients in roflumilast and placebo groups in trials M2–124 (C) and M2–125 (D). Error bars are SE. Reproduced from Calverley PMA et al Lancet 2009;374:685–94 with permission.
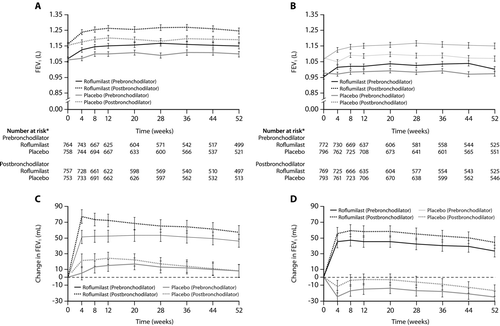
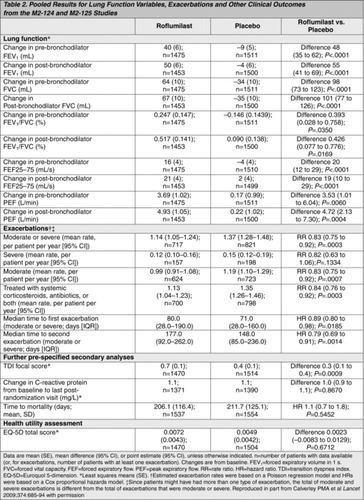
Table 3. Adverse events occurring in at least 2·5% of patients in one of the treatment groups of the M2–124 and M2–125 studies