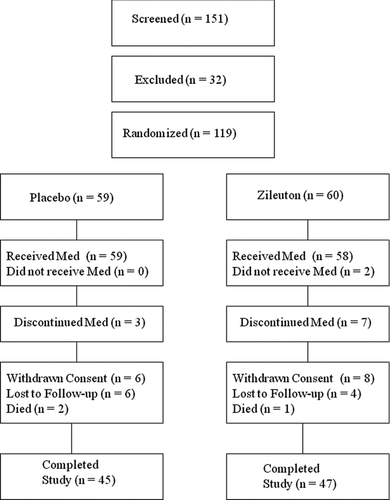
Figures & data
Figure 1 Study schematic. If patients remained hospitalized, they were visited as inpatients at the 7-, 14- and 30-day visits.
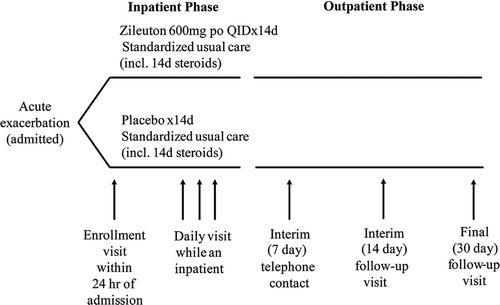
Table 1 Subject characteristics by intervention group
Table 2 Inpatient therapy by visit day
Figure 3 There was no statistically significant difference in hospital length of stay (the primary outcome) between groups (hazard ratio 0.96; 95% confidence interval [95% CI], 0.64–1.42).
![Figure 3 There was no statistically significant difference in hospital length of stay (the primary outcome) between groups (hazard ratio 0.96; 95% confidence interval [95% CI], 0.64–1.42).](/cms/asset/ccdfa417-aa6d-4d17-b464-7f0bede8f1ac/icop_a_540273_f0003_b.jpg)
Table 3 Treatment failure by intervention group
Table 4 Spirometric data at the 30-day visit
Figure 4 Urinary LTE4 and serum LTB4 levels. (A) Urinary LTE4 levels declined with zileuton as compared to placebo at 24 h (p<0.001) and 72 h (p = 0.006). (B) There was no statistically significant difference in the change in serum LTB4 levels at 30 days between treatment groups (p = 0.19), despite a non-significant decline in geometric mean LTB4 level of 40% with zileuton.
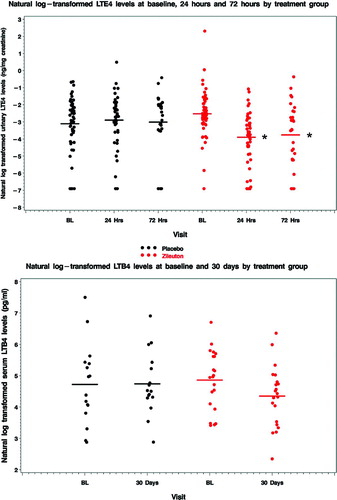
Table 5 Serious adverse events