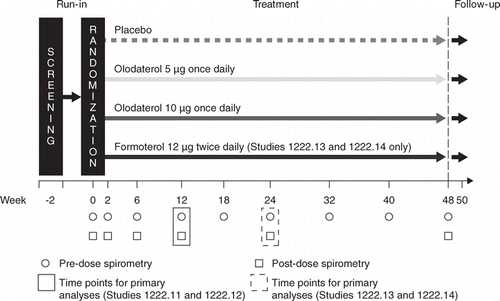
Figures & data
Table 1. Demographic and baseline patient characteristics by treatment group for 48-week, parallel-group trials (treated patient population)
Figure 2. AEs ≥2% in olodaterol 48-week studies in chronic obstructive pulmonary disease. On-treatment including 12-day washout period unless specified otherwise. AE = adverse event; GI = gastrointestinal.
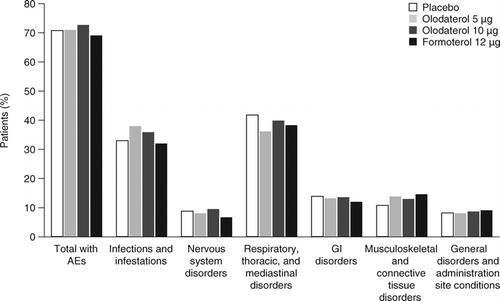
Table 2. Overview of AEs for all 48-week, olodaterol parallel-group trials
Table 3. Fatal AEs, adjudicated cause 48-week studies in COPD (on-treatment)
Table 4. Frequency of patients with MACE by treatment group in all 48-week studies
Table 5. Frequency of patients with AEs related to the cardiovascular system in all 48-week studies
Table 6. Incidence of cardiac and vascular AEs and serious AEs in patients with cardiac disorder at baseline or cardiac history
Table 7. Incidence of cardiac and vascular AEs and serious AEs in concomitant β-blocker medication subgroup