Figures & data
Figure 1. Summary of patient disposition in Study 1 (A) and Study 2 (B). AE, adverse event; FP/SAL, fluticasone propionate/salmeterol combination; ITT, intent-to-treat; PBO, placebo; PP, per protocol; UMEC, umeclidinium. *The run-in population included screening failures, run-in failures, and those in the ITT population (i.e., any patient who took at least one dose of open-label FP/SAL during the run-in period). Study 1: Reasons for withdrawal: PBO + FP/SAL: AE (n = 6), withdrew consent (n = 3), lost to follow-up (n = 2), protocol deviation (n = 4), lack of efficacy (n = 11), subject reached protocol-stopping criteria (n = 1); UMEC 62.5 + FP/SAL: PBO + FP/SAL: AE (n = 5), withdrew consent (n = 1), protocol deviation (n = 3), lack of efficacy (n = 5); UMEC 125 + FP/SAL: AE (n = 10), withdrew consent (n = 5), protocol deviation (n = 3), lack of efficacy (n = 3). Study 2: Reasons for withdrawal: PBO + FP/SAL: AE (n = 13), withdrew consent (n = 7), lost to follow-up (n = 1), protocol deviation (n = 2), lack of efficacy (n = 8); UMEC 62.5 + FP/SAL: AE (n = 10), withdrew consent (n = 8), protocol deviation (n = 1), lack of efficacy (n = 6); UMEC 125 + FP/SAL: AE (n = 6), withdrew consent (n = 4), lost to follow-up (n = 1), protocol deviation (n = 1), lack of efficacy (n = 6).
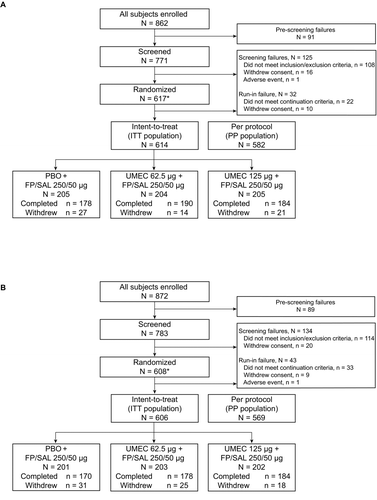
Table 1. Patient demographics and characteristics (ITT population) at screening and baseline
Table 2. Lung function, rescue use and HRQoL endpoints for both studies (ITT population)
Figure 2. LS mean (95% CI) change from baseline in trough FEV1 (L) in Study 1 (A) and Study 2 (B) (ITT). CI, confidence interval; FEV1, forced expiratory volume in 1 second; FP/SAL, fluticasone propionate/salmeterol combination; ITT, intent-to-treat; LS, least squares; PBO, placebo; UMEC, umeclidinium. Analysis performed using a repeated measures model with covariates of treatment, baseline (mean of the two assessments made 30 minutes and 5 minutes pre-dose on Day 1), smoking status, Day, Day by baseline and Day by treatment interactions.
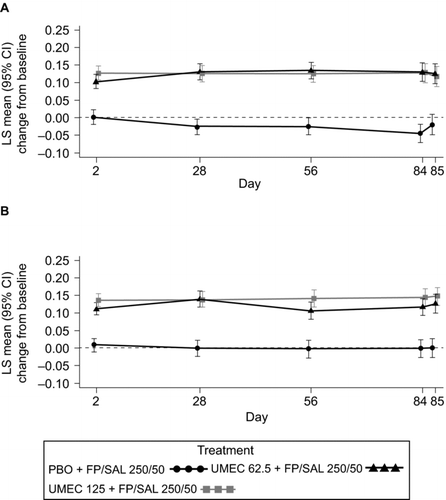
Figure 3. LS mean (95% CI) change from baseline in 0–6 hours WM FEV1 (L) in Study 1 (A) and Study 2 (B) (ITT). CI, confidence interval; FEV1, forced expiratory volume in 1 second; FP/SAL, fluticasone propionate/salmeterol combination; ITT, intent-to-treat; LS, least squares; PBO, placebo; UMEC, umeclidinium; WM, weighted mean. Analysis performed using a repeated measures model with covariates of treatment, baseline (mean of the two assessments made 30 minutes and 5 minutes pre-dose on Day 1), smoking status, Day, Day by baseline and Day by treatment interactions.
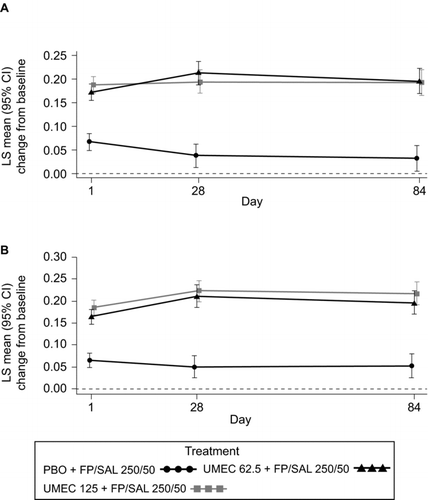
Figure 4. Serial (24 hours) FEV1 LS mean (95% CI) change from baseline on Day 1 and Day 84 (ITT population) in Study 1 (A) and Study 2 (B). CI, confidence interval; FEV1, forced expiratory volume in 1 second; FP/SAL, fluticasone propionate/salmeterol combination; ITT, intent-to-treat; LS, least squares; PBO, placebo; UMEC, umeclidinium. Analyses performed using a separate repeated measures model for each Day with covariates of treatment, baseline (mean of the two assessments made 30 minutes and 5 minutes pre-dose on Day 1), smoking status, time, time by baseline and time by treatment interactions.
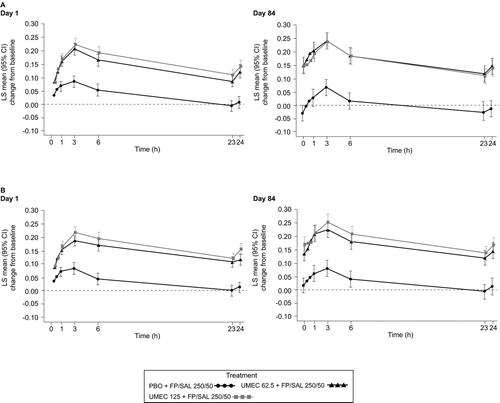
Table 3. Summary of on-treatment AEs reported by ≥ 3% of patients in any treatment group
