Figures & data
Figure 1. Desmosomal structure and composition. (A) Electron micrograph of a desmosome from mouse epidermis. The arrows demarcate the positions of the plasma membranes of neighboring cells. The brackets indicate the location of the cytoplasmic plaques. (B) Schematic representation of the proteins that assemble into desmosomes. Note that the transmembrane components (DSG and DSC) connect on the cytoplasmic surface of the desmosome with the plaque proteins (JUP, DSP and PKP) which in turn link to the intermediate filament (IF) cytoskeleton.
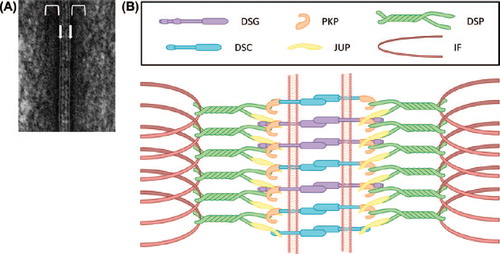
Figure 2. Clinical presentation of AEC patients. Scalp and palmar erosions on two patients affected by AEC. Patient images provided by the National Foundation for Ectodermal Dysplasias (NFED).
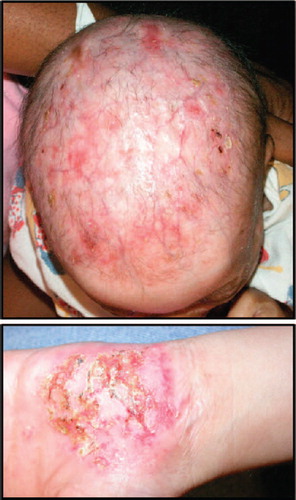
Figure 3. Focal downregulation of DSP and DSC3 expression in non-lesional skin of an AEC patient. (A,B) Normal human skin and (C, D) skin of an AEC patient. The dashed line demarcates the epidermal–dermal junction. The arrowheads in panels (C) and (D) point to areas of normal DSP and DSC3 staining, respectively, while the arrows indicate area in which expression of either DSC3 or DSP is significantly downregulated.
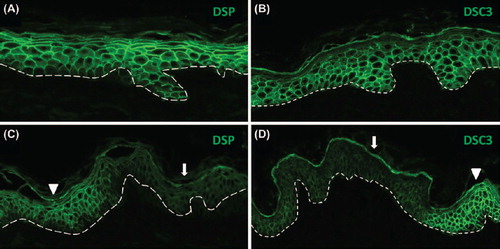
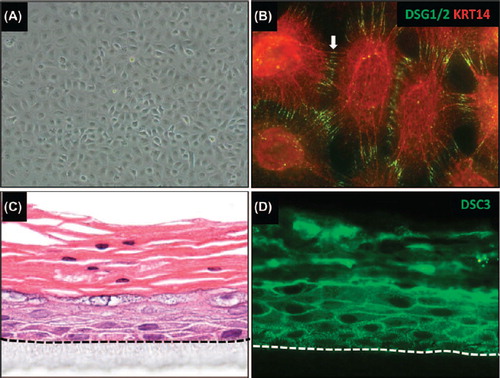
Figure 4. Generating in vitro tissue models using iPSC-derived keratinocytes. (A) iPSC-derived keratinocytes were generated following a protocol similar to a previously published procedure (CitationItoh et al., 2011). (B) iPSC-derived keratinocytes express keratin 14 (KRT14) and desmoglein 1/2 (DSG1/2). Note that these cells also express other epithelial markers, such as keratin 5, TP63, and α6β4 integrin (data not shown). (C) in vitro skin equivalent generated from human iPSC-derived keratinocytes on an artificial surface. Note that the epithelium stratified with a notable granular and cornified layer. Parakeratosis (retention of nuclei in the stratum corneum) occurs occasionally in in vitro skin equivalents. (D) Staining of the skin equivalent shown in (C) with an antibody for desmocollin 3 (DSC3). Note that these iPSC-derived skin equivalents also express other epithelial markers such as KRT5/14, KRT1/10, and loricrin (data not shown). The dotted line demarcates the junction between stratified epithelium and the artificial matrix on which the cells are growing.