Figures & data
Figure 1. The signaling mechanisms triggered by pemphigus autoantibodies reduce the cytoskeletal anchorage of desmosomes. Under resting conditions, desmogleins and desmocollins in desmosomes are linked to intermediate filaments via desmoplakin and the adaptor proteins plakoglobin and plakophilin. Desmosomal components interact with several signaling molecules: p38MAPK is associated with Dsg3, PKC is sequestered by keratin filaments via RACK1 and active RhoA is bound to extradesmosomal Dsg3. Upon autoantibody binding, p38MAPK is activated within the signaling complex with Dsg3, a process involved in EGF-R activation and RhoA inhibition, which finally causes keratin retraction. In parallel, PKC activation reduces intermediate filament binding to desmoplakin.
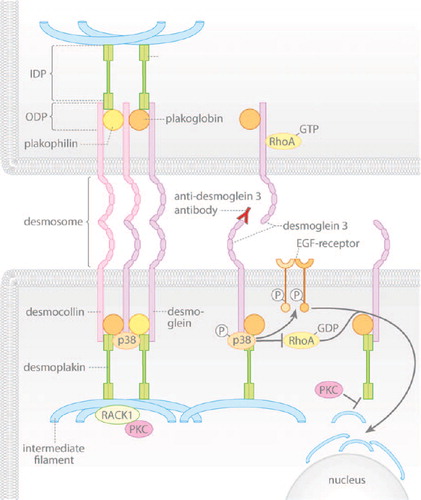
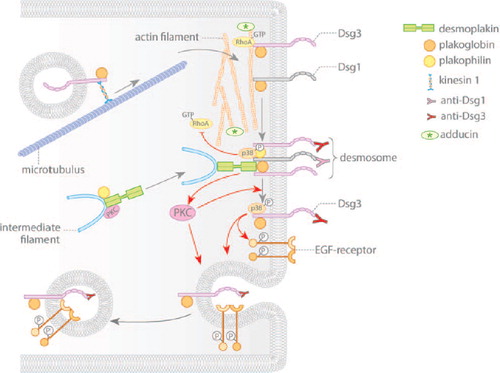
Figure 2. Pemphigus autoantibodies interfere with desmosomal cadherin turnover. Gray arrows indicate assembly and disassembly of desmosomal components. Red arrows denote changes in signaling induced by autoantibodies of pemphigus patients. Desmosomal cadherins are transported to the cell membrane in a microtubule-dependent process. Membrane localization of the desmosomal plaque is dependent on plakophilin and on PKC signaling. Pemphigus autoantibodies induce internalization and depletion of extradesmosomal Dsg3 via activation of p38MAPK and EGF-R. Furthermore, activation of PKC may be necessary for shifting Dsg3 out of the desmosome. Additionally, autoantibodies interfere with RhoA signaling, which primarily may impair assembly of desmosomes.