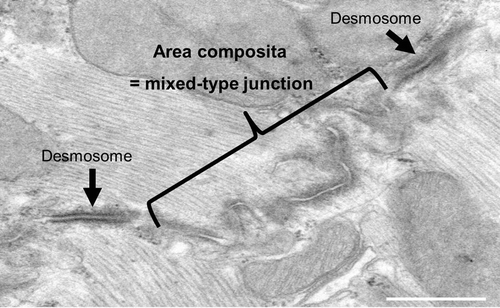
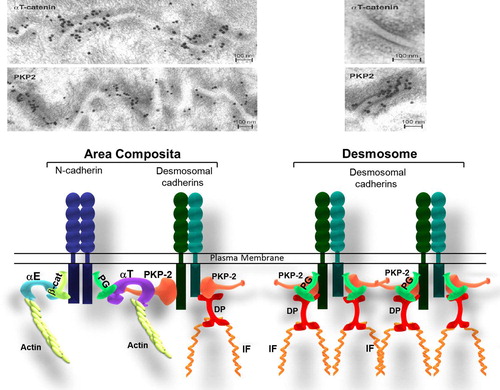
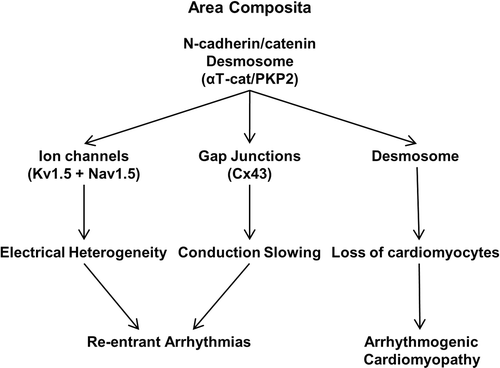
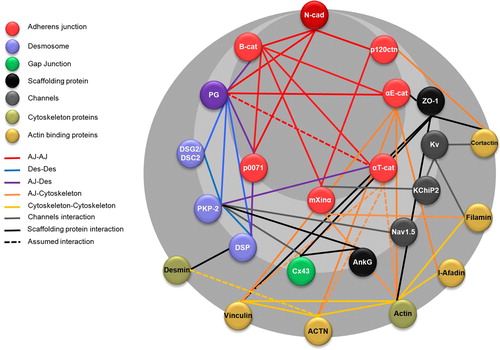
Figures & data
Table 1. Genetic manipulation of adherens junction proteins in the adult myocardium.