Figures & data
Table 1. Occurrence and type of infections observed in nonclinical studies of Compounds A, B, and C.
Figure 1. White blood cell (WBC) counts can be a biomarker of infection, but are generally not predictive, in monkeys presenting with epistaxis and upper respiratory effects in a toxicology study with Compound B. Elevations in WBC, when present, usually did not precede the onset of clinical signs. Monkeys #1–4 represent the vehicle control group; #5–8 the low-dose (1 mg/kg/day) group; #9–12 the intermediate-dose group (10 mg/kg/day); and #13–16 the high-dose group (50 mg/kg/day). Yellow-shaded bars represent the monkeys that presented clinically with epistaxis, with initial onset at Day 7 of treatment, and datapoints represent individual white-blood cell counts measured pre-dose [1 week before dosing (Day −7), blue diamonds], on Day 9 (after onset of clinical signs, pink squares), and on Day 13 (green triangles). Black horizontal line is the upper limit of laboratory’s historical control range for WBC counts (∼15,500). Infection-related findings included severe epistaxis with associated acute inflammation in nasal mucosa, decreased activity, dehydration, and/or pale mucous membranes; decreased food consumption or total inappetance; regenerative anemia; increased levels of acute-phase stress response proteins; gross and microscopic evidence of inflammation/septicemia; myeloid hyperplasia; and acute membra-noproliferative glomerulonephritis (consistent with a post-infectious event). Progression of these findings led to physical deterioration and euthanasia of one high-dose monkey on Day 10 and the remaining high-dose monkeys on Days 14 or 15.
![Figure 1. White blood cell (WBC) counts can be a biomarker of infection, but are generally not predictive, in monkeys presenting with epistaxis and upper respiratory effects in a toxicology study with Compound B. Elevations in WBC, when present, usually did not precede the onset of clinical signs. Monkeys #1–4 represent the vehicle control group; #5–8 the low-dose (1 mg/kg/day) group; #9–12 the intermediate-dose group (10 mg/kg/day); and #13–16 the high-dose group (50 mg/kg/day). Yellow-shaded bars represent the monkeys that presented clinically with epistaxis, with initial onset at Day 7 of treatment, and datapoints represent individual white-blood cell counts measured pre-dose [1 week before dosing (Day −7), blue diamonds], on Day 9 (after onset of clinical signs, pink squares), and on Day 13 (green triangles). Black horizontal line is the upper limit of laboratory’s historical control range for WBC counts (∼15,500). Infection-related findings included severe epistaxis with associated acute inflammation in nasal mucosa, decreased activity, dehydration, and/or pale mucous membranes; decreased food consumption or total inappetance; regenerative anemia; increased levels of acute-phase stress response proteins; gross and microscopic evidence of inflammation/septicemia; myeloid hyperplasia; and acute membra-noproliferative glomerulonephritis (consistent with a post-infectious event). Progression of these findings led to physical deterioration and euthanasia of one high-dose monkey on Day 10 and the remaining high-dose monkeys on Days 14 or 15.](/cms/asset/de05fa4c-5ab6-4278-8fee-0e32a3d6fd44/iimt_a_449745_f0001_b.gif)
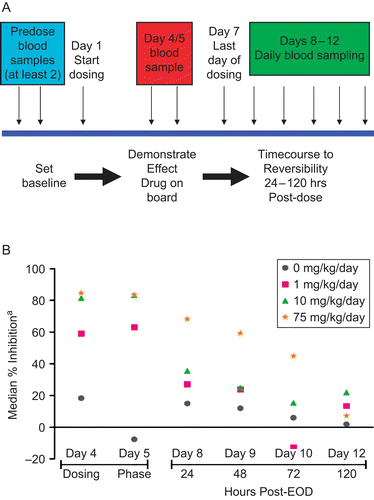
Figure 2. Ex vivo assessment of innate immune cell function in cynomolgus monkeys treated with Compound A for 1 week demonstrated a dose-dependent functional inhibition and time course to reversibility. (A) Study design. Compound A was administered at 1, 10, or 75 mg/kg/day for 1 week to 6 male monkeys/group followed by 24–120 hr of dose-free recovery. Blood samples (≈ 2 mL) were collected from the femoral vein of unanesthetized hosts into tubes containing sodium heparin as an anticoagulant twice during the pre-study period to establish baseline values, on Days 4 and 5 (≈ 1.5 hr after dosing), and at ≈ 24, 48, 72, and 120 hr after the final (Day 7) dose. Samples were analyzed upon collection for respiratory burst and phagocytosis function using commercially-available kits with known application to non-human primates. (B) Data were expressed as median % inhibition of the per day control group mean fluorescent intensity (MFI). MFI were normalized to pre-test MFI values for each monkey before percentage inhibition calculation.