Figures & data
Figure 1. Inhibition of splenocyte proliferation by TiO2 nanoparticles after in vitro exposures. Splenocytes were prepared from naive C57BL/6 mice. (A) Splenocytes were cultured with various concentrations of TiO2 nanoparticles and cell viability then measured using an MTT assay. (B and C) Splenocytes were cultured with various concentrations of TiO2 nanoparticles in the presence of (B) lipopolysaccharide (LPS, 25 µg/ml) or (C) concanavalin A (ConA, 2 µg/ml) for 48 h. Cells were then pulsed with 1 µCi [3H]-thymidine/well for 4–6 h before collection onto nitrocellulose filters. Total [3H]-thymidine incorporated into cells was measured (as cpm) via a scintillation counter. (D) Bone marrow-derived macrophages (BMDM) from naïve mice were stimulated with LPS in the presence of various concentrations of TiO2 nanoparticles for 48 h. Nitric oxide (NO) production in the supernatant was then measured by the Griess reaction. Each bar shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Values significantly (*P < 0.05, **P < 0.01) different from LPS- or ConA-stimulated control (receiving medium only) counterpart cultures [as applicable] are indicated.
![Figure 1. Inhibition of splenocyte proliferation by TiO2 nanoparticles after in vitro exposures. Splenocytes were prepared from naive C57BL/6 mice. (A) Splenocytes were cultured with various concentrations of TiO2 nanoparticles and cell viability then measured using an MTT assay. (B and C) Splenocytes were cultured with various concentrations of TiO2 nanoparticles in the presence of (B) lipopolysaccharide (LPS, 25 µg/ml) or (C) concanavalin A (ConA, 2 µg/ml) for 48 h. Cells were then pulsed with 1 µCi [3H]-thymidine/well for 4–6 h before collection onto nitrocellulose filters. Total [3H]-thymidine incorporated into cells was measured (as cpm) via a scintillation counter. (D) Bone marrow-derived macrophages (BMDM) from naïve mice were stimulated with LPS in the presence of various concentrations of TiO2 nanoparticles for 48 h. Nitric oxide (NO) production in the supernatant was then measured by the Griess reaction. Each bar shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Values significantly (*P < 0.05, **P < 0.01) different from LPS- or ConA-stimulated control (receiving medium only) counterpart cultures [as applicable] are indicated.](/cms/asset/0a5016f5-0c5a-4535-8372-3d0d3177534d/iimt_a_543995_f0001_b.gif)
Figure 2. Inhibition of ex vivo lymphocyte development due to exposure to TiO2 nanoparticles. Mice were intraperitoneally (IP) injected with 10 mg TiO2 nanoparticles/kg once a day for 7 days. (A) Splenocytes and (B) thymocytes from each animal were prepared from organs harvested 24 h after the final TiO2 injection. One million cells from each organ were then incubated on ice for 30 min with fluorescent-labeled antibodies to the indicated markers. The cells were then washed twice with RPMI 1640-5% fetal bovine serum (FBS) and then analyzed via flow cytometry. Each population was determined and sizes compared between the control and TiO2 nanoparticle-injected groups. Each bar shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Value significantly (*P < 0.05, **P < 0.01) different from the control group.
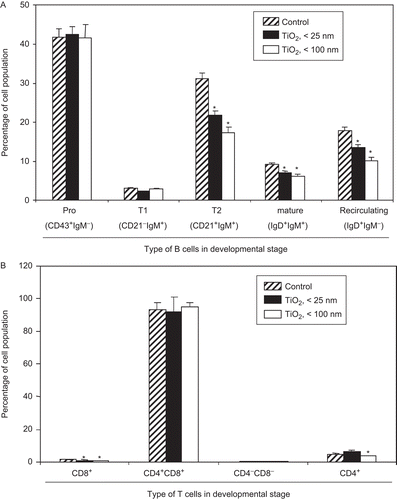
Figure 3. LPS-stimulated ex vivo splenocyte proliferation was reduced by host exposure to nanoparticle TiO2. Mice were IP injected with 10 mg TiO2 nanoparticles/kg once a day for 7 days. (A) Splenocytes from control and TiO2-injected mice were prepared from organs harvested 24 h after the final TiO2 injection and total spleen cell number determined. (B) One million isolated splenocytes/host were incubated with anti-B220 and anti-CD3 antibodies to permit flow cytometric determination, respectively, of B- and T-lymphocyte levels. Each population was determined and sizes compared between the control and nanoparticle-injected groups. Numbers inside each bar indicate percentage of each cell type in the total population analyzed. (C) Splenocytes were cultured with LPS (25 µg/ml) or ConA (2 µg/ml) for 48 h and cell proliferation then measured using an MTT assay. Each bar shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Value significantly (*P < 0.05, **P < 0.01) different from that of the control group.
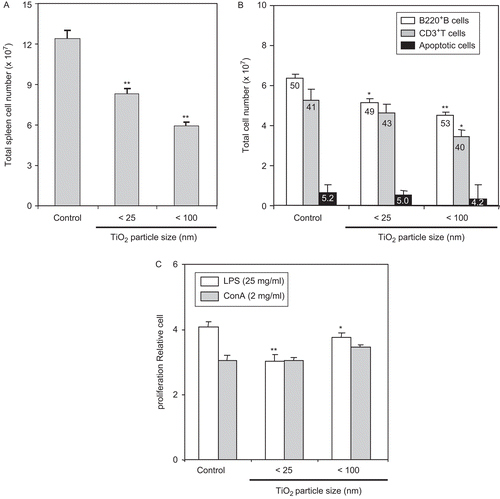
Figure 4. Inhibitory effect of TiO2 nanoparticles on cytokine production. Mice were IP injected with 10 mg TiO2 nanoparticles/kg once a day for 7 days. Peritoneal cells were then collected from the peritoneal cavity of each mouse (with RPMI 1640 medium) 24 h following the final TiO2 injection. (A) Cells were stimulated with LPS for 48 h and culture supernatants were then collected for analyses of TNF-α (top), IL-6 (middle), and IL-1β (bottom) levels by enzyme-linked immunosorbent assay (ELISA). Data shown are in terms of mean ± SE. Value significantly (**P < 0.01) different from the control group. (B) Splenocytes from these control and TiO2 nanoparticle-injected mice were prepared and reacted with fluorescein isothiocyanate (FITC)-labeled anti-CD11b antibodies for 30 min. The cells were then washed twice with RPMI 1640-5% FBS and then analyzed via flow cytometry. Each population was determined and sizes compared between the control and TiO2 nanoparticle-injected groups. Each bar shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Value significantly (**P < 0.01) different from that of the control group.
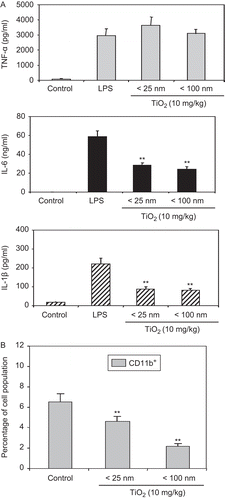
Figure 5. Measurement of NK1.1+/CD11b+ cells in spleens of mice pre-exposed to TiO2 nanoparticles. Mice were IP injected with 10 mg TiO2 nanoparticles/kg once a day for 7 days. Splenocytes from control and TiO2-injected mice were prepared from organs harvested 24 h after the final TiO2 injection and reacted with FITC-labeled anti-CD11b or PE-labeled anti-NK1.1 antibodies for 30 min. The cells were then washed twice with RPMI 1640-5% FBS and each cell analyzed via flow cytometry. Each population was determined and sizes compared between the control and TiO2 nanoparticle-injected groups. Each bar shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Value significantly (*P < 0.05, **P < 0.01) different from that of the control group.
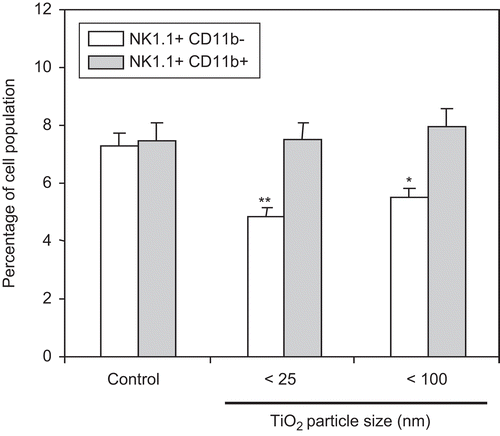
Figure 6. Tumor growth was increased by host pre-tumor implantation exposure of TiO2 nanoparticles. Five mice per group were IP injected with 10 mg TiO2 nanoparticles/kg once a day for 28 days. B16F10 mouse melanoma cells were then subcutaneously implanted 24 h after the final nanoparticles injection; control mice were injected with vehicle only. Tumor size was then measured daily with Vernier calipers for 12 days. (A) Body weight of each animal was measured daily. (B) Tumor volume was calculated by the multiplication of the [(long axis)/2] × (short axis)2. The line graph shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Value significantly (*P < 0.05, **P < 0.01) different from that of the control group.
![Figure 6. Tumor growth was increased by host pre-tumor implantation exposure of TiO2 nanoparticles. Five mice per group were IP injected with 10 mg TiO2 nanoparticles/kg once a day for 28 days. B16F10 mouse melanoma cells were then subcutaneously implanted 24 h after the final nanoparticles injection; control mice were injected with vehicle only. Tumor size was then measured daily with Vernier calipers for 12 days. (A) Body weight of each animal was measured daily. (B) Tumor volume was calculated by the multiplication of the [(long axis)/2] × (short axis)2. The line graph shown is a representative of three experiments performed. Data shown are in terms of mean ± SE. Value significantly (*P < 0.05, **P < 0.01) different from that of the control group.](/cms/asset/1e5408f1-74f4-493f-9c86-b2412a15249f/iimt_a_543995_f0006_b.gif)
