Figures & data
Figure 1. Flow cytometry analysis of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled cells. Lymphocytes were labeled with CFSE before stimulation with concanavalin A (ConA) in the presence or absence of valproic acid (VPA). One of three independent experiments is shown. M1: Percentage of parent population; M2: percentage of divided cells.
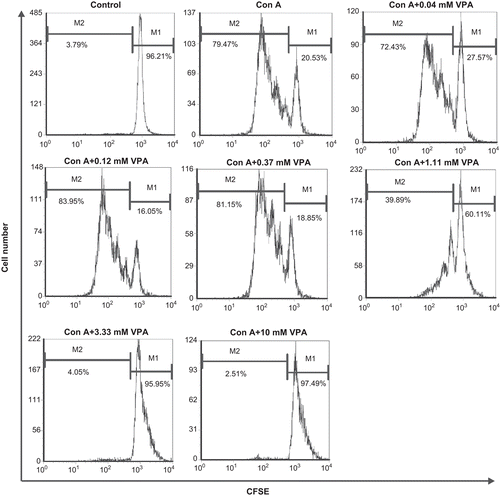
Table 1. Effect of valproic acid (VPA) on CD69 surface expression by activated lymphocytes stimulated with concanavalin A (ConA) for 72 h.
Figure 2. Effect of valproic acid (VPA) on CD69 expression on the cell surfaces of activated lymphocytes. Cells were stimulated with concanavalin A (ConA) for 72 h in vitro in the presence or absence of VPA. CD69 surface expression on the cells was determined by flow cytometry. Data are one of three independent experiments.
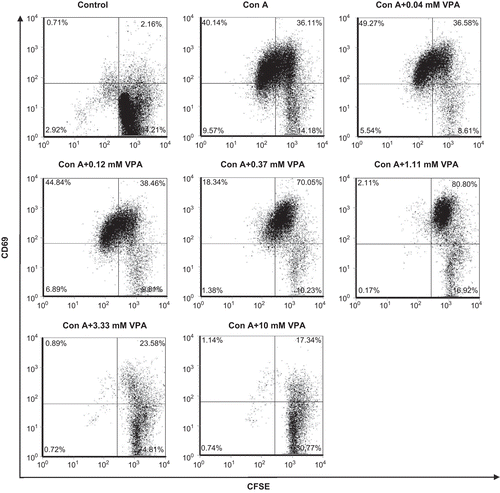
Table 2. Effect of valproic acid (VPA) on cell cycle distribution and apoptosis of activated lymphocytes stimulated with concanavalin A (ConA) for 72 h.
Figure 3. Effect of valproic acid (VPA) on cell cycle distribution and apoptosis of activated lymphocytes. Cells were stimulated by concanavalin A (ConA) in the presence or absence of VPA for 72 h, stained with propidium iodide, and analyzed by flow cytometry. Data are one of three independent experiments. Cell cycle phases are indicated above each mark.
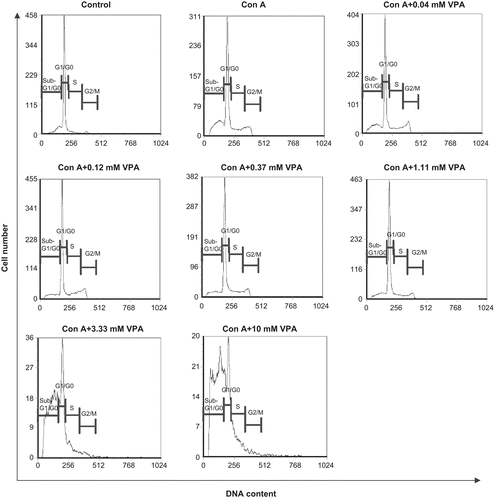
Figure 4. Effect of valproic acid (VPA) on apoptosis-related proteins Bax and Bcl-2 expression in concanavalin A (ConA)-activated lymphocytes. Lymphocytes were stimulated with ConA in the presence or absence of VPA for 1 and 24 h. Bax and Bcl-2 levels were then assessed by western blotting. β-Tubulin was used as a loading control. Data, expressed as a ratio to the loading control, are from one of three independent experiments. The level of immunoreactivity was measured as peak intensity using AlphaEaseFC software.
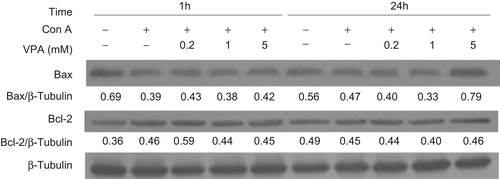
Figure 5. Effect of valproic acid (VPA) on the expression of acetylated histone H3 (acetyl-H3) and phosphorylated H2A.X (γH2A.X) in concanavalin A (ConA)-stimulated lymphocytes. Cells were stimulated with ConA in the presence or absence of VPA for 24 h. γH2A.X and acetyl-H3 levels were then assessed by Western blotting. Total histone H3 was used as a loading control. Data are expressed as a ratio to the loading control. All blots represent data from three independent experiments.
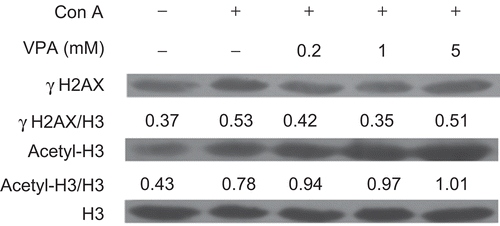
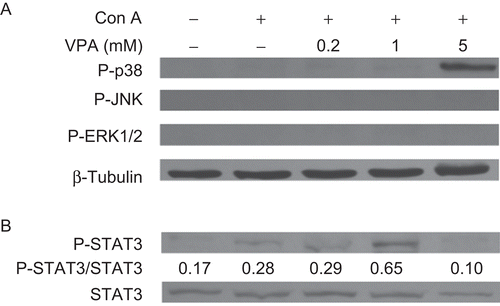
Figure 6. Effect of valproic acid (VPA) on the phosphorylation of mitogen-activated protein kinases (MAPKs) (A) and STAT3 (B) in concanavalin A (ConA)-stimulated lymphocytes. Cells were stimulated with ConA in the presence or absence of VPA for 72 h. Whole-cell lysates were then analyzed by Western blotting for the levels of p38, ERK1/2, and JNK phosphorylation (P-p38, P-ERK1/2, and P-JNK) and phospho-STAT3 (P-STAT3). STAT3 and β-tubulin levels were used as the loading controls. Data are expressed as a ratio to the loading control. All blots represent data from one of three independent experiments.