Figures & data
Table 1. Effects on serum corticosterone, body weight, and organ indices in mice treated orally with PFOS daily for 7 days.
Table 2. Distribution apoptotic and necrotic cells in spleens from mice treated daily with PFOS orally for 7 days.
Figure 1. Effects of PFOS on DNA fragmentation (mono- and oligonuclesomes). Thymocytes and splenocytes were isolated from male C57Bl/6 mice following oral exposures to PFOS for 7 days. DNA fragmentation was determined by Cell Death Detection ELISA. Data are presented as mean (± SE); n = 12/group. When significant differences were detected by the F-test (p < 0.05), a Dunnett’s t-test was used to compare treatment groups to the control group. *Value significantly different from control at p ≤0.05.
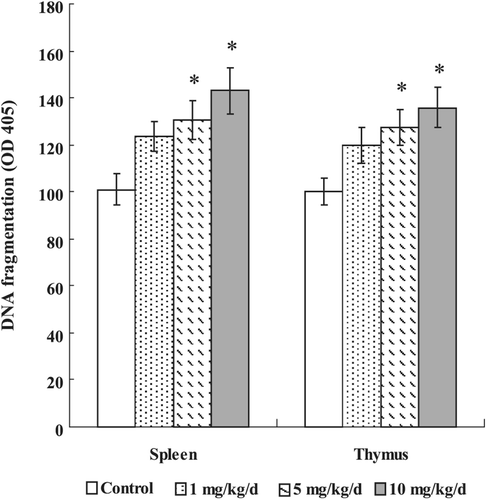
Figure 2. Effects of PFOS on mitochondrial membrane potential. Thymocytes and splenocytes were isolated from male C57Bl/6 mice following oral exposures to PFOS for 7 days. Rh-123 was added and the cells were incubated for 60 min. Fluorescence was measured using a flow cytometer with an FL-1 filter. Data are presented as mean (± SE); n = 12/group. When significant differences were detected by the F-test (p < 0.05), a Dunnett’s t-test was used to compare treatment groups to the control group. *Value significantly different from control at p ≤ 0.05.
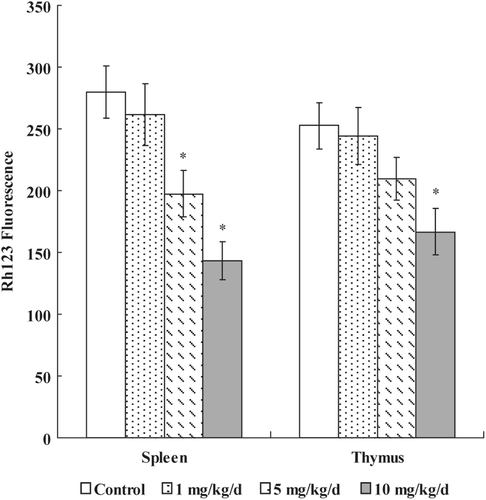
Figure 3. Effects of PFOS on generation of ROS. Thymocytes and splenocytes were isolated from male C57Bl/6 mice following oral exposures to PFOS for 7 days. DCFH-DA was added and the cells were incubated for 60 min. DCF fluorescence was measured using a flow cytometer with an FL-1 filter. Data are presented as mean (± SE); n = 12/group. When significant differences were detected by the F-test (p < 0.05), a Dunnett’s t-test was used to compare treatment groups to the control group. *Value significantly different from control at p ≤ 0.05.
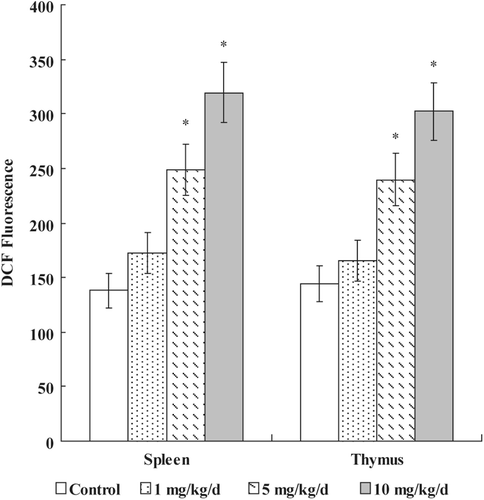
Table 3. Anti-oxidative enzyme activity and GSH content in splenocytes from mice treated daily with PFOS orally for 7 days.
Table 4. Expression of bcl-2, p53, bax, caspase-3, and caspase-9 proteins in splenic lymphocytes from mice treated daily with PFOS orally for 7 days.