Figures & data
Table 1. The phenotypic markers present on the surface of mouse Langerhans’ cells, mouse Langerin-positive (+ve) dermal DC and mouse Langerin-negative (−ve) DC.
Figure 1. Changes in DC marker expression following activation. In the resting state, DC express high levels of CCR1, CCR2, CCR5, CCR6, and E-cadherin. Upon activation, these markers are down-regulated and a number of other markers are up-regulated, including CCR7, CD40, ICAM1, MHC-II, and the co-stimulatory complex (CD40, CD80, and CD86). In addition, metalloproteinase (MMP)-2, MMP-3, MMP-9, and the cytokines IL-1β, IL-6, and IL-18 are all released. These changes facilitate mobilization, maturation, and migration of DC away from the skin and towards the LN.
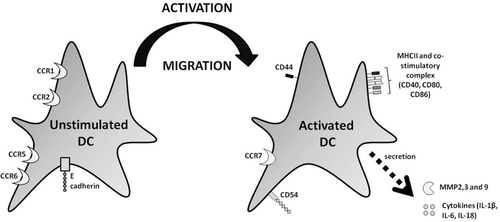
Figure 2. The interaction between the TLR and NLR signaling pathways. Upon stimulation by a variety of danger signals, different TLR activate various signaling pathways. Ligands for TLR1, -2, -5, -6, -7, -8, -9, and -10 all activate MyD88-dependent pathways, whereas ligands for TLR3 and 4 activate both MyD88 and TRIF-dependent pathways. Ultimately, activation of both the MyD88 and TRIF-dependent pathways causes the induction of NF-κB translocation. This translocation facilitates transcription of numerous pro-inflammatory proteins including pro- IL-1β, pro-IL-18, and pro-IL-33. In order for these proteins to be secreted and, thus, in order for the proteins to have physiological effect, they must be activated. This activation is achieved through cleavage by caspase-1. Caspase-1 itself must be activated, and this requires the formation of the inflammasome. The formation of the inflammasome is also induced by a number of different danger signals. Thus, it is held that activation of DC via the TLR signaling pathway requires activation of the inflammasome as well.
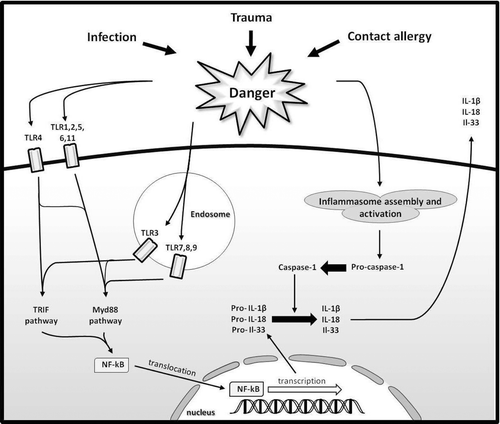
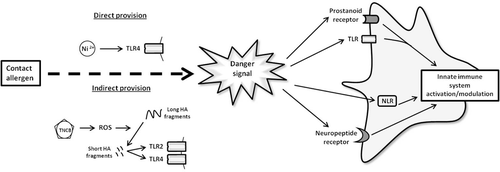
Figure 3. Linking danger signals and contact sensitization. The current review suggests that contact allergens induce the provision of danger signals via two routes, i.e., either directly or indirectly. An example of direct provision is observed in nickel sensitization. Here, nickel acts directly to bind TLR4 and activate the TLR4 pathway. Alternatively, an example of indirect provision is observed in sensitization to TNCB. In this, TNCB induces the production of ROS. These ROS cause the breakdown of long hyaluronic acid (HA) fragments into short HA fragments. The short HA fragments are capable of activating TLR2 and TLR4, thus it is held that TNCB activates DC via this mechanism.