Figures & data
Figure 1. Comparative immunophenotyping of human and cynomolgus macaque CD4+ T-cells. Analysis gated on CD4+ T-cells shown in top panels and on CD8+ T cells shown in bottom panels. Human and cynomolgus macaque naive (N) and central memory (CM) CD4+ and CD8+ T-cells are CCR7+CD28+. Human CD4+ effector memory (EM) T-cells are CCR7−CD28+ in contrast to cynomolgus macaque CD4+ EM T-cells, which are CCR7−CD28−. Cynomolgus macaque CD4+CCR7−CD28+ T-cells are a transitional sub-set with CM properties. Both human and cynomolgus macaque CD8+ N and CM T-cells express CD28 compared to CD8+ EM T-cells that lack CD28 expression.
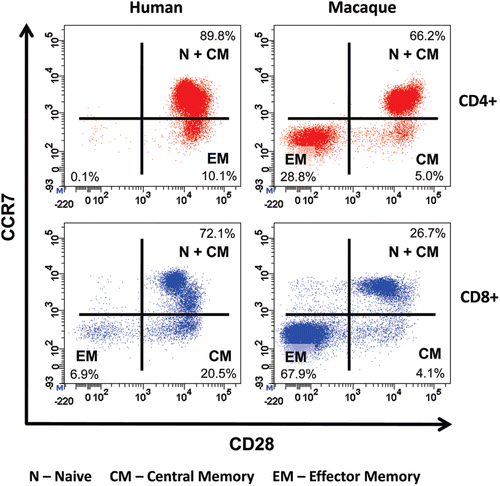
Figure 2. Proliferation of PKH26-stained human PBMC incubated 3 days with (a) 1 μg/well aqueous TGN1412 vs (b) 1 μg/well immobilized TGN1412. Lymphocyte proliferation appears as bands of cells with decreasing PKH26 fluorescence as dye is divided equally between daughter cells and increased forward scatter (FSC) corresponding to increased size due to blasting. No proliferation is observed with aqueous TGN1412 (a) compared to a strong proliferative response with immobilized TGN1412 (b). Low power light microscope images of 2 × 105 human PBMC in tissue culture after 3 days culture with (c) 1 μg/well aqueous TGN1412 vs (d) 1 μg/well immobilized TGN1412.
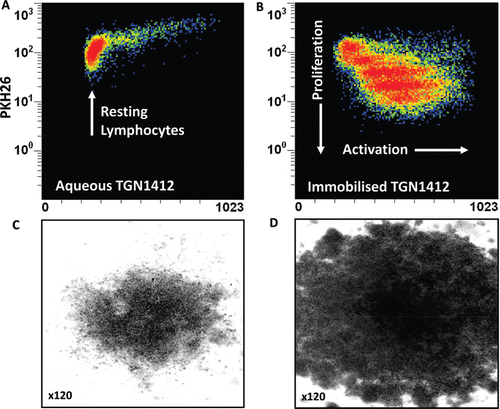
Figure 3. Cytokine release from human PBMCs stimulated in vitro for 24, 48, and 72 h with 1 µg/well immobilized isotype-matched control (IgG4κ), Rituximab (IgG1), Campath-1H (IgG1), and TGN1412 (IgG4κ). The lectin PHA at 10 µg/ml was used as mitogen control. Cytokine release into culture supernatants was measured by ELISA. (a) TNFα, (b) IL-8, (c) IFNγ, and (d) IL-2 release are expressed in pg/ml. Donors responses shown (n = 9). * Denotes p < 0.05 (one-way analysis of variance with bonferroni correction).
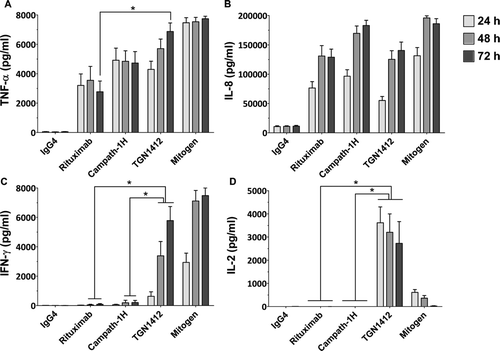
Figure 4. Intracellular IFN-γ staining of human PBMC stimulated for 24 h with 1 μg/well of immobilized TGN1412, Rituximab, Muromonab, and Campath-1H. CD4+ T cells, CD8+ TT cells and NK cells are identified using the key above. TGN1412 stimulates IFN-γ secretion from CD4+ T-cells, Rituximab, and Campath-1H stimulate IFNγ secretion from NK cells and CD8+ T-cells and Muromonab stimulate IFN-γ secretion from CD4+ and CD8+ T-cells and NK cells.
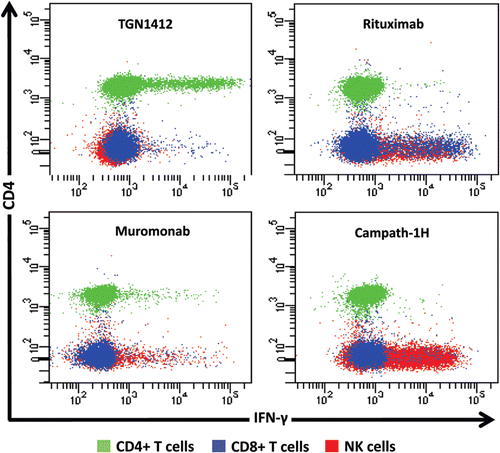
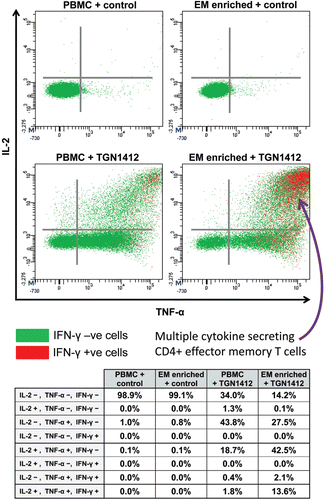
Figure 5. Intracellular TNFα, IL-2, and IFNγ staining of human PBMCs and effector memory T-cell (EM) enriched PBMC following stimulation with either 1 µg/well immobilized IgG4 isotype matched control mAb or TGN1412. Analysis shown is gated on CD3+CD4+ T-cells. Cells in the right hand quadrants are positive for TNFα, cells in the upper quadrants are positive for IL-2, cells positive and negative for IFN-γ are identified using the key above.Cells in the upper right corner in red are positive for TNFα, IL-2, and IFNγ. The frequency of cytokine positive cells is expressed as a percentage of CD3+CD4+ T-cells. Quadrant statistics for cytokine positive CD3+CD4+ T-cells are shown in the table below.