Figures & data
Figure 1. (A) Pulmonary Il23a and (B) Il17a mRNA abundance, determined by qRT-PCR, in WT mice exposed to room air or ozone (0.3 ppm) for 72 h. Results shown are means ± SE from 5–6 mice/group and are expressed relative to the air exposed mice. *p < 0.05 vs air.
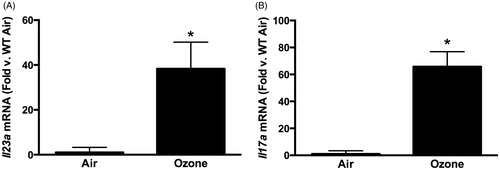
Figure 2. (A) Pulmonary Il17a mRNA abundance (expressed relative to in WT mice). (B) Pulmonary IL-17A+ γδ T-cells. (C) Pulmonary IL-17A+ interstitial macrophages measured by flow cytometry in WT and IL-23−/− mice exposed to ozone (0.3 ppm for 72 h). Results shown are means ± SEM of 8–9 mice/group for qRT-PCR and four mice/group for flow cytometry. *p < 0.05 vs WT mice.
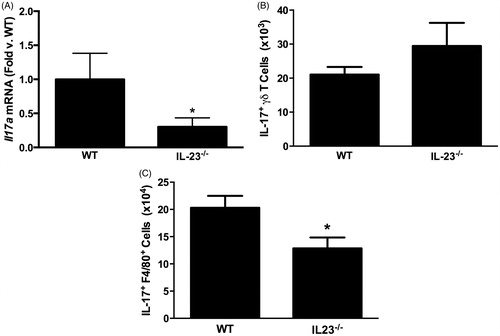
Figure 3. Bronchoalveolar lavage (BAL) (A) neutrophils and (B) macrophages in WT and IL-23−/− mice exposed to ozone (0.3 ppm for 72 h). Results shown are means ± SE of 8–9 mice/group. (C) Log %BAL neutrophils plotted vs ΔΔCt values for Il17a. All mice were ozone-exposed. The regression line shown was calculated from the combined data from the WT and IL-23−/− mice. Note: increase in ΔΔCt indicates reduced Il17a expression.
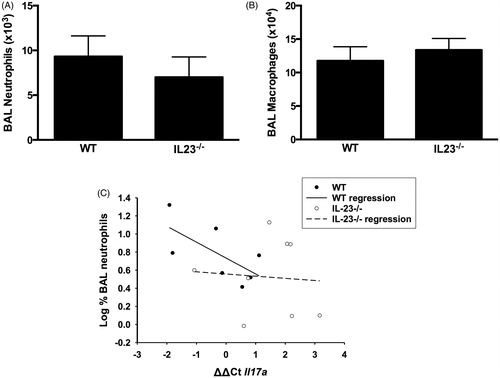
Figure 4. Pulmonary (A) Il17a and (B) Il23a mRNA abundance (expressed relative to WT mice) and BAL (C) neutrophils, (D) macrophages and (E) total protein in WT mice exposed to ozone (0.3 ppm for 72 h) that had been treated with anakinra or saline. Results shown are means ± SE of 6–12 mice/group. *p < 0.05 vs saline-treated mice.
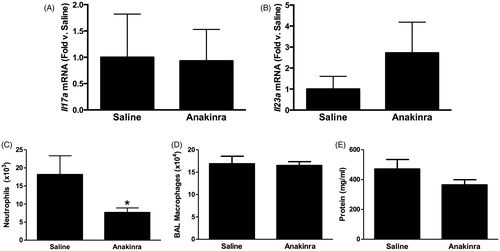
Figure 5. BAL (A) LIF, (B) IL-6 and (C) GM-CSF assayed by multiplex from WT (C57BL/6J) mice exposed to ozone (0.3 ppm for 72 h) that had been treated with anakinra or saline. Results shown are means ± SE of eight mice/group. * p < 0.05 vs saline-treated mice.
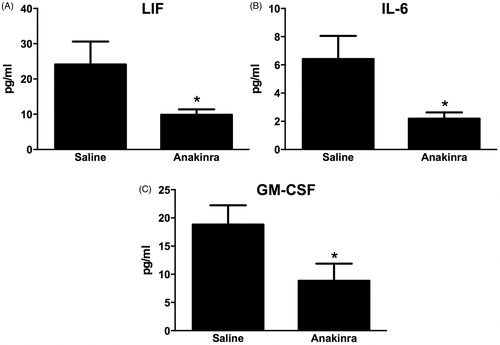
Figure 6. (A) Gating strategy used to assess lung DC. Cells were first gated based on forward scatter (FSC) and side-scatter (SSC) characteristics. Cells positive for MCH-II and CD11c were determined to be either dendritic cells (DC, SiglecF−) or macrophages M∅, SiglecF+). (B) Total number of MHC-II+/CD11c+/SiglecF− DC in WT mice exposed to air or ozone (0.3 ppm) for 24, 48 or 72 h. (C) Total number of MHC-II+/CD11c+/SiglecF− DC in WT and Flt3l−/− mice exposed to air or ozone (0.3 ppm) for 72 h. Results shown are means ± SE of 7–8 mice/group. *p < 0.05 vs air-exposed mice with same genotype; #p < 0.05 vs WT mice with same exposure.
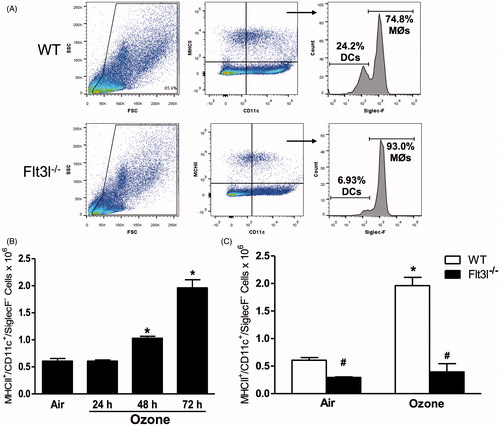
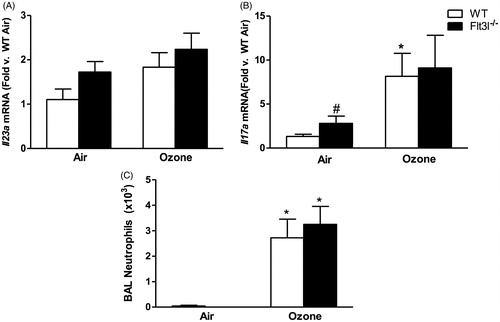
Figure 7. Pulmonary (A) Il23a and (B) Il17a mRNA abundance (expressed relative to WT air mice) and (C) BAL neutrophil numbers in WT and Flt3l−/− mice exposed to room air or ozone (0.3 ppm) for 72 h. Results shown are means ± SE of 7–8 mice/group. *p < 0.05 vs air-exposed mice with same genotype; #p < 0.05 vs WT mice with same exposure.