Figures & data
Figure 1. Macrophage viability in terms of Stimulation Index (SI% = 100% × [OD test/OD control]). Viability of macrophages (MQ) were assessed after 48 h culture in various test systems and in the presence/absence of test (indicated) exogenous agents (n = 5 cultures/treatment). Data shown are means ± SD. LPS: Lipopolysaccharide; Dex: Dexamethasone; BSA: Bovine Serum Albumin; Saf: Safranal; A. muc: Alyssum mucilage.
![Figure 1. Macrophage viability in terms of Stimulation Index (SI% = 100% × [OD test/OD control]). Viability of macrophages (MQ) were assessed after 48 h culture in various test systems and in the presence/absence of test (indicated) exogenous agents (n = 5 cultures/treatment). Data shown are means ± SD. LPS: Lipopolysaccharide; Dex: Dexamethasone; BSA: Bovine Serum Albumin; Saf: Safranal; A. muc: Alyssum mucilage.](/cms/asset/55d0f657-ff4f-4c5d-a2e4-7864935bee29/iimt_a_1139642_f0001_c.jpg)
Figure 2. Phagocytic activity. Macrophages (MQ) were cultured/treated using the 2-D and 3-D conditions and then phagocytic activity was evaluated via measures of phagocytosis of FITC-labeled NP after a 6 h period. (A) Representative light microscopic evaluation of cells in a 3-D system (NP only, no additional MQ treatments); Magnification 20×. (B) Corresponding representative fluorescent microscopic evaluation; Magnification 20×. (C) NP uptake using flow cytometry. Representative histograms derived from dot-plots show there an enhanced phagocytic activity among MQ held on collagen-chitosan scaffolds compared to by MQ in 2-D plates (with all exogenous treatments). Abbreviations are as in legend to . Relative percentages of NP+ cells (means ± SD; n = 5 replicates/analysis) for a given sample are in each histogram.
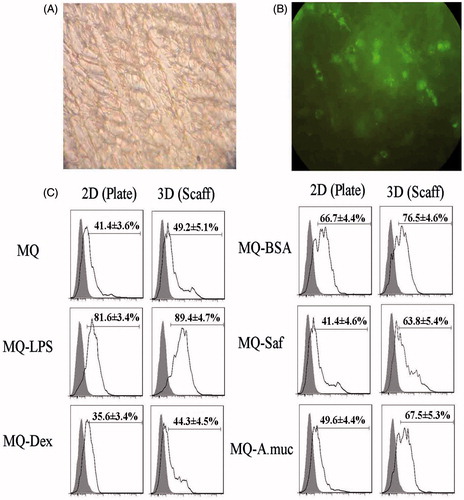
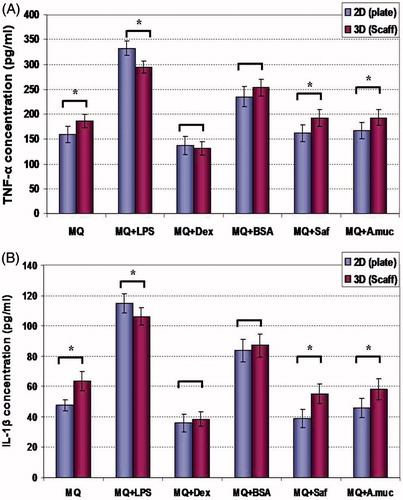
Figure 3. TNFα and IL-1β production. Effects of different treatment/maintenance systems on macrophage TNFα and IL-1β production over 48-h period are shown. Values are mean (pg/ml) ± SD from n = 5 replicates/regimen. *Value significantly different as a function of maintenance system for any given cell treatment is indicated (p < 0.05). Abbreviations are as in legend to . For each cytokine, treatment vs treatment analyses of significant differences within a defined maintenance system were not performed.