Figures & data
Figure 1. The majority of CNS in the first three month of life is caused by the following genes: The NPHS1 gene codes for nephrin and is responsible for the Finnish type CNS; the NPHS2 gene codes for podocin and leads to familial focal segmental sclerosis. Less common is a WT1 gene mutation causing DDS or Frasier syndrome. The LAMB2 gene is also associated with CNS and leads to Pierson syndrome. Other infrequent mutations in PLCE1, LMX1B (responsible for Nail–Patella syndrome) and LAMB3 have also been described. A small part is attributed to a nongenetic etiology with CNS due to congenital syphilis, congenital toxoplasmosis, congenital CMV infection or neonatal autoantibodies against endopeptidase.
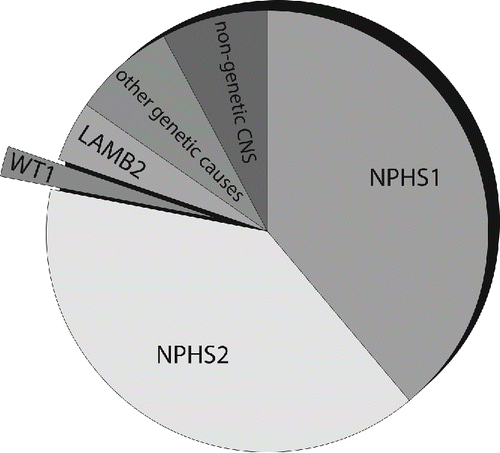
Figure 2. Location of the proteins responsible for the majority of CNS: Nephrin (NPHS1 gene) is a transmembrane protein of the immunoglobulin superfamily and interacts through its C-terminal part with podocin (NPHS2 gene), a harpin-like scaffolding protein both localized in the slit diaphragm (SD) in between the podocytic processes. WT1 encodes a zinc-finger transcription factor that plays a key role during kidney and genital development. Laminin beta 2 (LAMB2 gene) is a component of the glomerular basement membrane (GBM).
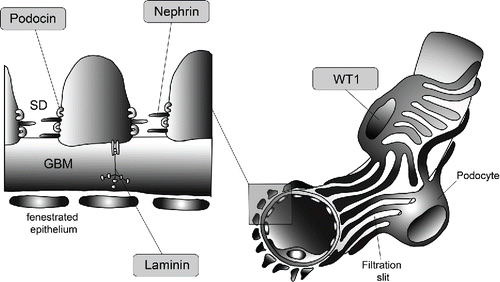
Figure 3. Histological, macroscopic, and electron microscopy observations from postmortem examination. a: Enlarged hyperlobulated kidney with macroscopically small cystic change at the corticomedullar transition zone. b: Uterus didelphys (**) with duplicated cervix and a single vagina. Normal development of rectum (*). c: Histology of the kidneys with glomeruli showing diffuse mesangial sclerosis and hypercellularity. There is marked interstitial fibrosis and tubular atrophy with formation of microcystic tubule dilatations and a lymphocytic inflammation (H.E. staining). d: Electron microscopy of the kidney shows extensive effacement of podocytes with fatty vacuoles.
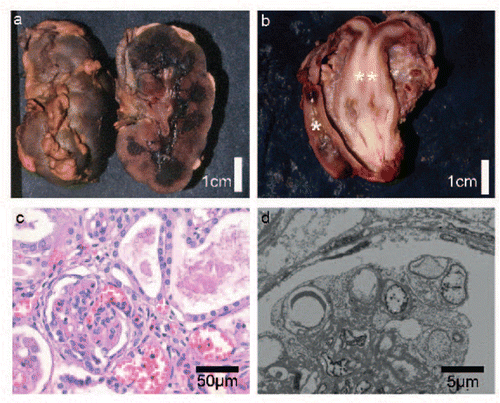
Table 1. Cases reported with c.1097G>A (p.(Arg366His)) mutation in the WT1 gene.
