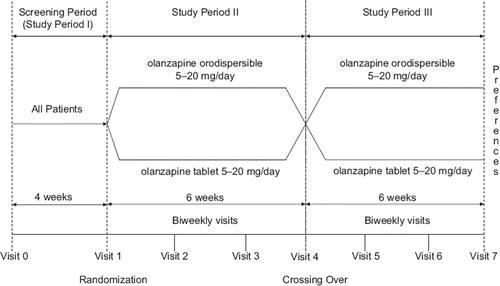
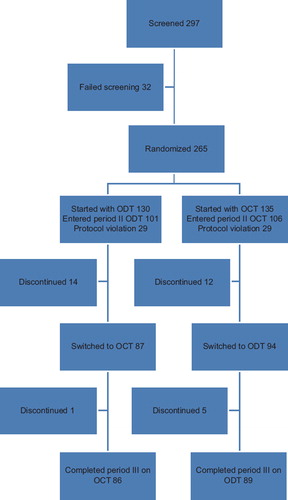
Figures & data
Table I. Demographic characteristics of patients in primary and secondary analyses
Table II. Patient preference for olanzapine formulation by treatment sequence.
Table III. Number of patients with adverse events by treatment and study period (safety population).