Figures & data
Scheme 1. Overview over the experiment. (1) (a) The particles (FWF, UFWF, Fe2O3 and Fe3O4) were suspended in water. (b) Nasal lavage fluids were concentrated and desalted. (2) Each particle type was incubated with nasal lavage proteins for 6 h. (3) Proteins bound to the particles (the protein corona) were separated from unbound proteins by centrifugation. The pellet was either (a) dissolved in water and the proteins were trypsin digested or (b) incubated with denaturing solution. (4) The proteins in the supernatant were trypsin digested. (5) The tryptic peptides from steps 4 and 3b were analyzed with LC-MS/MS. (6) The denatured proteins were separated on 2DE, gel slices were cut out, the proteins were digested with trypsin, and the peptides were identified with MALDI-TOF MS.
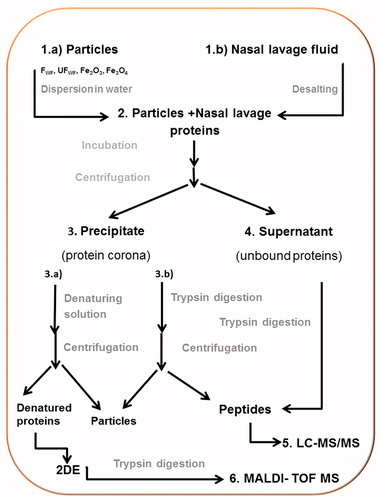
Figure 1. Airborne welding fume particles. Log-normal distribution to the average number mobility size distribution of airborne agglomerated welding particles detected with SMPS.
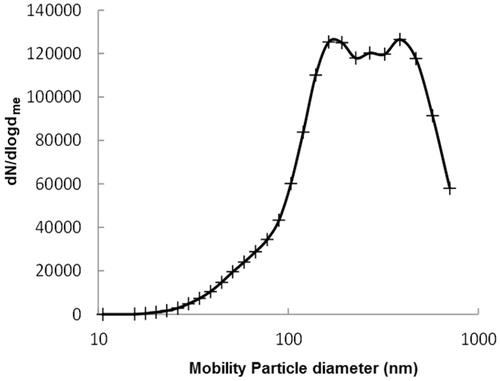
Table 1. Size increase (mean diameter) between particles suspended in water and following addition of nasal lavage proteins or secretory leukocyte peptidase inhibitor (SLPI).
Figure 2. Comparison of protein-corona patterns from different particle, 50 μg of the bound proteins were separated on 2DE. (A) FWF, (B) UFWF, (C) Fe2O3, and (D) Fe3O4. (1) PLUNC, (16) lysozyme C, (7) keratine and (2) lipocalin 1. Each sample was analyzed in triplicates. The most representative gels are presented here. Numbers refer to identified proteins listed in Table S3.
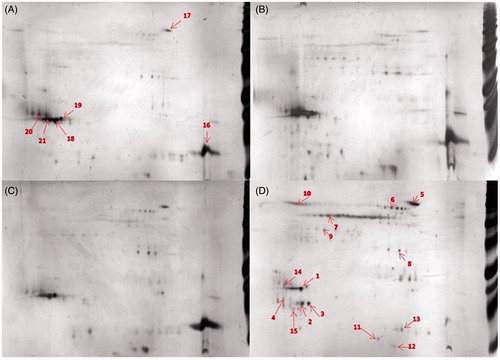
Table 2. Proteins with high affinity for the particles.
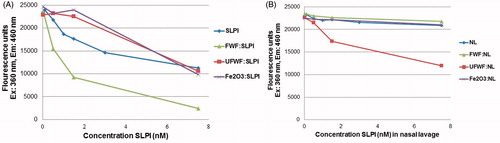
Figure 3. HNE substrate inhibition. Detection of SLPI inhibition of HNE activity was performed with (A) free SLPI compared with SLPI:FWF, SLPI:UFWF, and SLPI:Fe2O3 protein corona complexes and (B) nasal lavage protein (NL, which contains endogenous SLPI) compared with NL:FWF, NL:UFWF, and NL:Fe2O3 protein corona complexes.