Figures & data
Figure 1. Electronmicrograph of control, pairfed, and zinc deficient groups after 2 weeks. The scale bar = 2 µm. (A) Electronmicrograph of 2ZC group testes illustrating normal features of basement membrane (BM) showing basal lamina (bl), collagen fibres (cf), and endothelial cells (ed). Leydig cell nucleus (Nu) revealed characteristic peripheral heterochromatin (Hc). Presence of Type-A Spermatogonia (Sg) with its distinctive spherical nucleus and dense nucleolar body in association with the inner aspect of the nuclear envelope (Ne) is evident along with a large number of mitochondria (mt) with tubular cristae, rough endoplasmic reticulum (rER), ribosomes (r) and lysosomes (Ly) in the cytoplasm. (B) Electronmicrograph of 2PF group testes showing disrupted basement membrane (BM) with basal lamina (bl), collagen fibres (cf), and myoid cell process (My). Sertoli cell nucleus (Sc) exhibiting indentation and cytoplasm showing a large number of multivesicular bodies (mvb), lipid droplets (Ld), dilated smooth endoplasmic reticulum (sER), rough endoplasmic reticulum (rER), inter-Sertoli cell junctional complexes (Is), and parts of spermatocyte nuclei (Sp) with synaptonemal threads (St). (C) Ultrastructure of testes of 2ZD group testes displaying wavy basement membrane (BM) with basal lamina (bl), endothelial cells (ed), and disrupted collagen fibres (cf). Cytoplasm showing extensive vacuolization (V) and disruption of intercellular bridges (Icb), polyribosomes (pr), ribosomes (r), smooth endoplasmic reticulum (sER), rough endoplasmic reticulum (rER), abundant multivesicular bodies (mvb), and displaced Type A spermatogonium nucleus (Nu) showing chromatin conglomerates (Ch) with distinct nuclear envelope (Ne). (D) Ultrastructure of primary spermatocyte nuclei (Sp) of 2ZD group testes illustrating early apoptotic change viz. chromatin condensation (Cc) on the periphery. Extensive cytoplasmic damages with a large number of multivesicular bodies (mvb) are also distinct. (E) Electronmicrograph of 2ZD group testes illustrating juxtanuclear region of cytoplasm showing extensive vacuolization (V), dilated smooth endoplasmic reticulum (sER), and aggregation of multivesicular bodies (mvb). Peripheral heterochromatin (Hc) of Leydig cell nucleus (Nu) are evident.
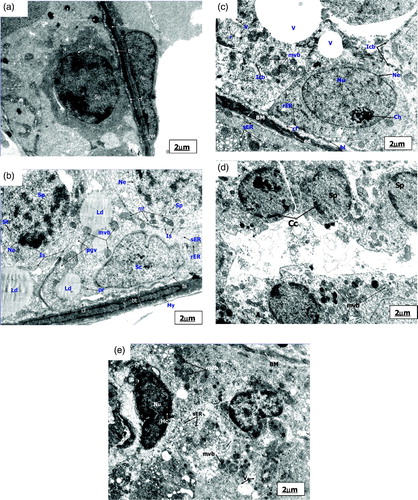
Figure 2. Electronmicrograph of control and pairfed groups after 4 weeks. The scale bar = 2 µm. (A) Electronmicrograph of testes of 4ZC group illustrating normal features of basement membrane (BM) with basal lamina (bl), collagen fibres (cf), and endothelial cells (ed). Cytoplasm shows characteristic peripheral arrangement of mitochondria (pmt) with dilated intra-cristal space, rough endoplasmic reticulum (rER), and inter-Sertoli cell junctional complexes (Is). Sertoli cell (Sc) and Leydig cell (Lc) are distinct. The nucleus of Leydig cells (Lc) reveals peripheral heterochromatin (Hc). (B) Electronmicrograph of testes of 4PF group showing basement membrane (BM) with basal lamina (bl), collagen fibres (cf), and endothelial cells (ed). Sertoli cell (Sc) with lobulated nucleus (Nu), mitochondria (mt), smooth endoplasmic reticulum (sER), lysosomes (Ly), phagocytic vacuoles (pgv), and mutivesicular bodies (mvb) seen in large number in the cytoplasm.
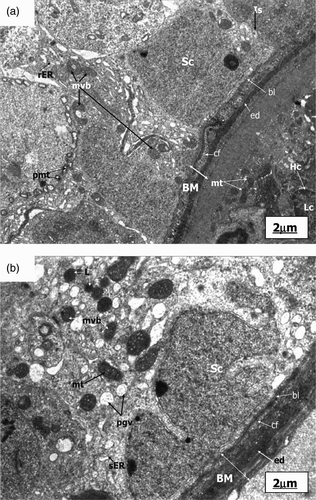
Figure 3. Electronmicrograph of zinc deficient groups after 4 weeks showing several apoptotic features. The scale bar = 2 µm (A, B, C, D, E, and G); The scale bar = 1 µm (F and H). (A) Electronmicrograph of testes of 4ZD group showing basement membrane (BM) with layer of basal lamina (bl), collagen fibers (cf) and cytoplasmic extensions of endothelial cells (ed). Degenerative changes are evident in the Sertoli cell (Sc) cytoplasm. Multivesicular bodies (mvb), vacuolization (v), and few mitochondria (mt) are distinct. (B) Electronmicrograph of 4ZD group testes showing disrupted spermatogenic epithelium (BM), dissolution of collagen fibres (cf) and displaced Type B spermatogonium nucleus (Nu) with condensed chromatin (Cc) associated with the nuclear envelope (Ne). Cytoplasm reveals vacuolization (V) with complete loss of junctional complexes. Lysosomes (Ly), deformed mitochondria (Amt), polyribosomes (pr), smooth endoplasmic reticulum (sER), and lipid droplets (Ld) are evident. (C) Ultrastructure of 4ZD group testes displaying primary spermatocyte nucleus (Nu) showing dissolution of nuclear envelope (Ne) and chromatin conglomerates (Ch). Cytoplasm reveals ovoid mitochondria (mt) and interrupted intercellular bridges (Icb). Cytoplasmic dissolution (CD) is also evident in some areas. (D) Electronmicrograph of testes of 4ZD group displaying detachment of acrosomal cap (ac) from head region of spermatid nucleus (Nu) just above the sub-acrosomal space (sas). One of the apoptotic spermatid (Asd) showing ring of condensed chromatin (Cc) around the nuclear periphery. Presence of mitochondria (mt) and lysosomes (Ly) are distinct. Degeneration of cytoplasm (CD) between the two spermatids is evident. (E) Electronmicrograph of testes of 4ZD group revealing deformed sperm heads (DS) with condensed nucleus (Nu), and disintegrated nuclear envelope (Ne). Cytoplasm shows few deformed mitochondria (Amt), multivesicular bodies (mvb), and dissolution of cytoplasm (CD) in some areas. (F) Cross sectional view of mid-piece (mp), principal piece (pp), and end piece (ep) of 4ZD spermatozoa tail revealing outer dense fibres (df) and central fibres (cf), mitochondrial sheath degeneration (mts) and retention of superfluous cytoplasm (sfc). (G) Aggregation of spermatozoa enclosed by a cytoplasm and common membrane (M). (H) Electronmicrograph of testes of 4ZD group displaying late apoptotic stage of Leydig cell nucleus (Nu) in which both chromatin (Cc) and cytoplasm have condensed. Peripheral heterochromatin (Hc) is distinct. Blebs (B) in the nuclear membrane of Leydig cell is also evident.
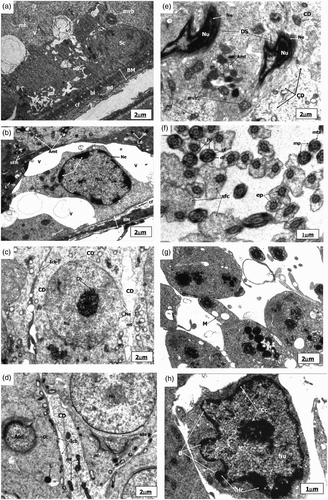
Figure 4. Microphotograph of TUNEL staining after 2 weeks for control, pairfed, and zinc deficient groups. Note: 400X. (A) Microphotograph of testes of 2ZC group revealing negative TUNEL staining in all the germ cells. (B) Microphotograph of testes of 2PF group showing few TUNEL positive germ cells (arrows). (C) Microphotograph of testes of 2ZD group exhibiting few TUNEL positive apoptotic spermatogonia and primary spermatocytes (arrows).
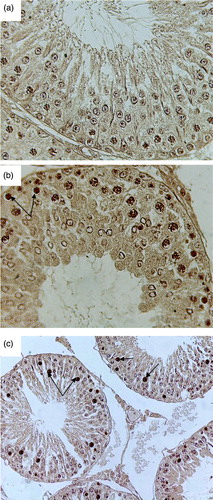
Figure 5. Microphotograph of TUNEL staining after 4 weeks for control, pairfed, and zinc deficient groups. Note: 400X. (A) Microphotograph of testes of 4ZC group exhibiting negative TUNEL staining in all the germ cells. (B) Microphotograph of testes of 4PF group showing few TUNEL positive cells (arrows). (C) Microphotograph of testes of 4ZD group revealing increase in the number of apoptotic cells. Positive staining seen in spermatogonia, primary spermatocytes, and giant cells (Gi) (cap-phase spermatids) (arrows).
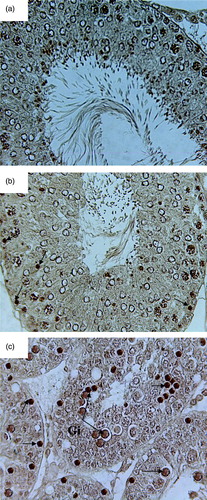
Table 1. Apoptotic Index (%) in testes of Wistar rats.
Table 2. Epididymal sperm concentration (x 106/ ml) and motility (%) of Wistar rats.
Table 3. Fertility Index of male Wistar rats.