Figures & data
Figure 1. Types of annexin V-FITC and propidium iodide staining. Annexin V-FITC and propidium iodide staining enables the identification of apoptotic, necrotic, and viable populations. Their mechanism of action is highlighted. Scale bar represents 10 µm.
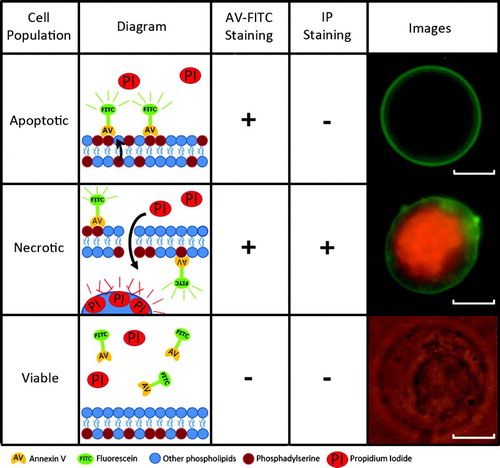
Table 1. Results from enzymatic disaggregation and specific apoptotic-/necrotic-staining observed in 13 of 17 patients with optimized protocol. (Four samples were used for the optimization.)
Figure 2. Flow cytometry separation of cells. A) Forward Scatter (FS) / Side Scatter (SS) window. Polygon R1 selects cells from the whole population in order to discard cell fragments (left) and aggregates (right). The subsequent graphs relate to polygon R1. B1) Non-stained control, whole population without labeling. B2) PI single-stained control, with the necrotic population in red and the non-stained population in blue. B3) FITC single-stained control, with the AV-FITC stained population in green and the non-stained population in blue. C) Study population (Annexin V-FITC plus propidium iodide). Apoptotic (R2 in green), viable (R3 in blue), and necrotic (R4 in red) populations are clearly identified. The selected populations correspond to the green (R2) and blue (R3) polygons in C.
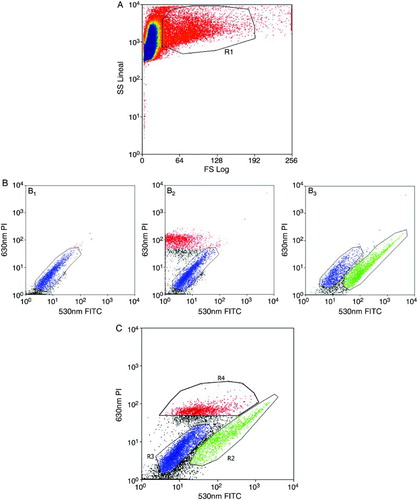
Table 2. Flow cytometry results obtained from 3 out of 14 patients with optimized parameters. (Eleven samples were used for the optimization.)
Figure 3. Triple-color FISH. The hybridization of centromeric probes for chromosomes X (green), Y (red), and 18 (blue) to apoptotic and viable cell nuclei are shown. Images A – C are from the apoptotic fraction. Images D – F are from the viable fraction. Scale bar represents 10 µm. (SC: Sertoli Cells; Sg: Spermatogonium; Pp: Prophase; Ss: Secondary Spermatocyte; St: Spermatid; Sz: Spermatozoon).
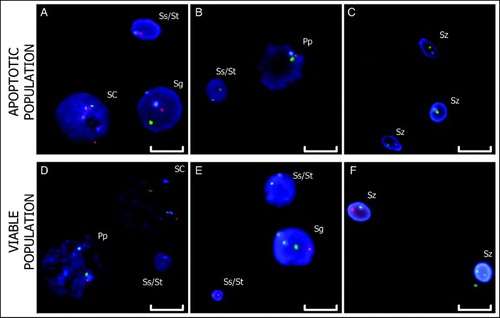
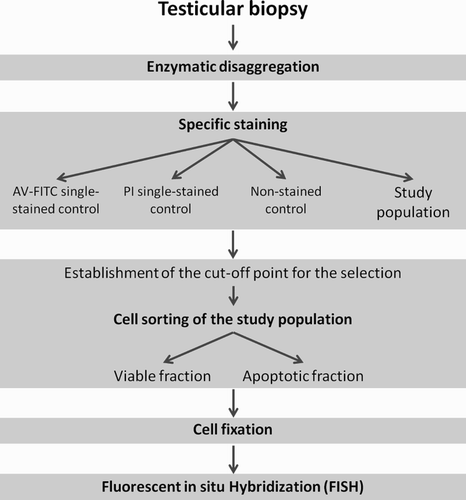
Figure 4. Scheme of the sequential methodology. The methodology developed consists of five steps: 1) enzymatic disaggregation of testicular tissue, 2) specific staining of apoptotic cells, 3) cell sorting by flow cytometry, 4) cell fixation, and 5) FISH (PI: propidium iodide; AV-FITC: Annexin V conjugated with FITC).