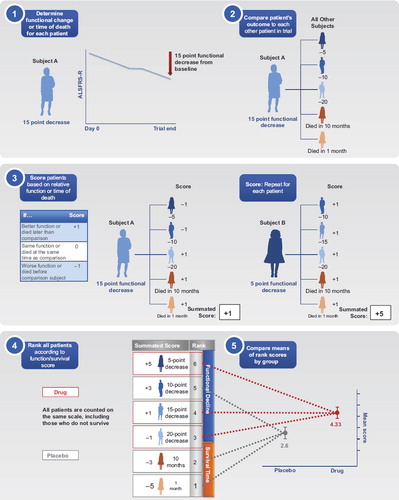
Figures & data
Table I. Examples of published composite endpoints and analyses for clinical trials.
Table II. Dexpramipexole phase II, part 2 study results (Citation19).
Table III. Power of EMPOWER study based on simulation of various hypothetical scenarios for outcome of ALSFRS-R and survival.