Figures & data
Figure 1. The rate of disease progression in participants at the time of trial enrolment. Calculated as (40-enrolment ALSFRS)/months from symptom onset.
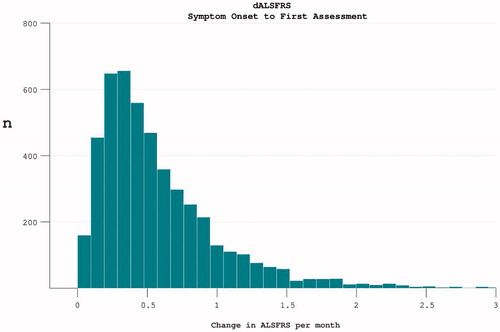
Figure 2. The spread of disability levels as measured by ALSFRS at both the time of trial enrolment and at the last available time-point (trial termination) for each individual.
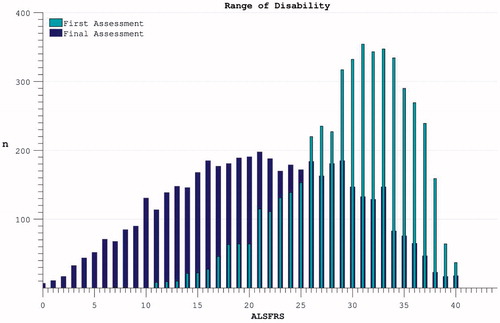
Figure 3. Accumulation of disability over time appears to slow over the course of the trial (dotted line), but declining numbers of participants are noted over time. Progression within groups of survivors to defined end-points appears more linear.
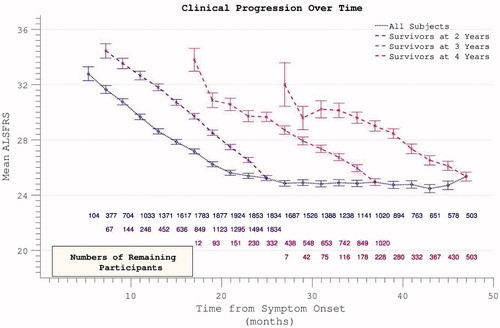
Figure 4. Decline in FVC mirrors ALSFRS in demonstrating apparent slowing across all participants but linear decline within survivor sub-groups.
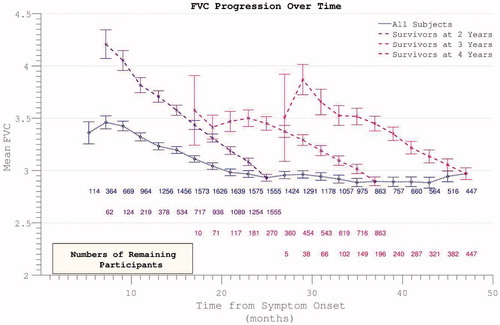
Figure 5. The rate of disease progression during the trial (calculated between assessment intervals) does not alter if analysis restricted to survivors. Including all participants (dotted line), the rate of disease progression appears to fall as more rapidly progressive patients drop out.
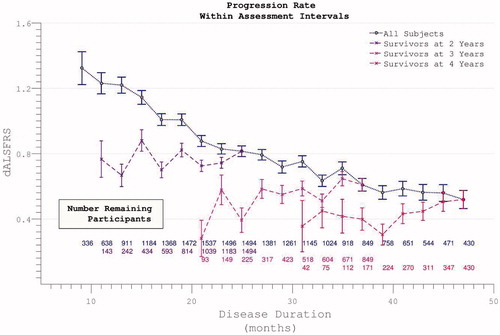
Figure 6. The rate of disability accumulation over the course of the trial, ignoring the time-period from symptom onset to trial enrolment.
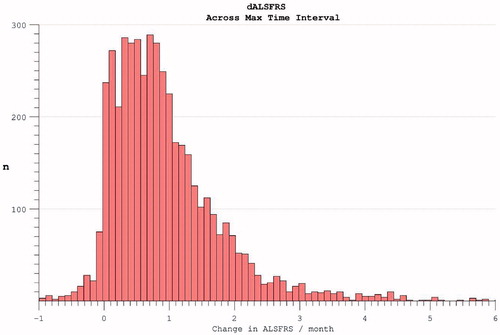
Figure 7. The time-difference (median = 5.8 months) between reported symptom onset and our estimate of when ALSFRS = 40 (based on individual ALSFRS gradient during trial). Later enrolment allows more accurate prognostication for an individual, as more time has passed for disability accumulation.
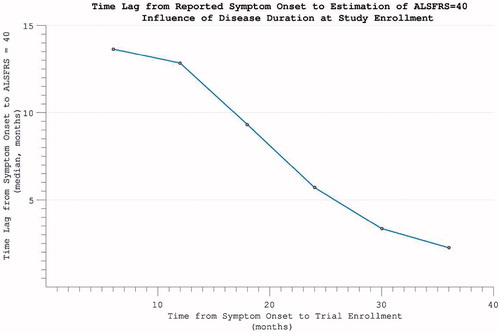
Figure 8. Predicting disease time-course from symptom onset. Within individuals with more rapidly progressive disease, using the symptom onset to enrolment rate to predict disease duration was highly accurate. For individuals with slower disease progression, greater inaccuracy is introduced.
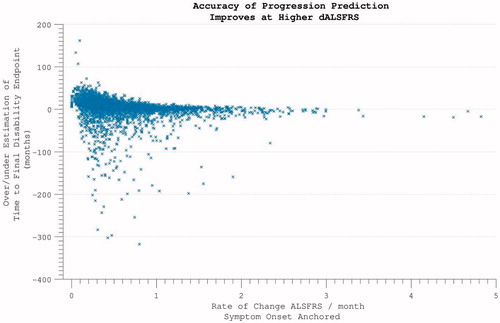
Figure 9. Hazard ratio of progression to significant disability (ALSFRS < = 21, the median final ALSFRS recorded) appears to plateau and diminish with time. Complicated by declining trial participation.
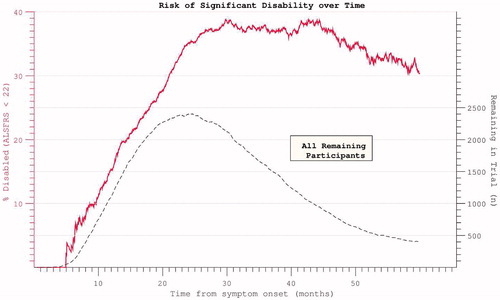
Figure 10. Hazard ratios for both disability and death appear to plateau despite restricting analysis to participants with mortality data at census.
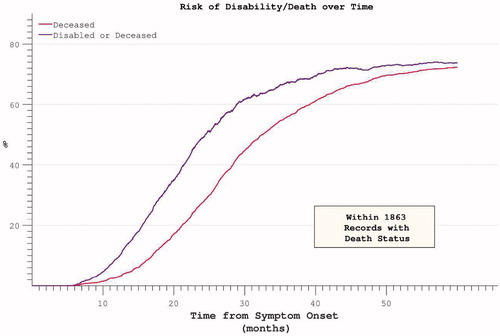
Figure 11. FVC and ALSFRS correlations with mortality, measured as initial absolute values, initial rates and interval rates. Initial rates predict mortality most accurately.
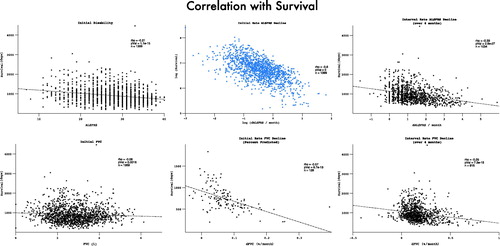
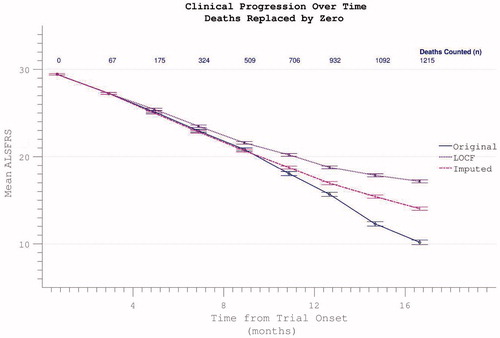
Figure 12. Possible dropout mitigation strategies include last observation carried forward (LOCF) or imputation of the missing values using prior δALSFRS. Imputation maintains linear progression of disability accumulation.
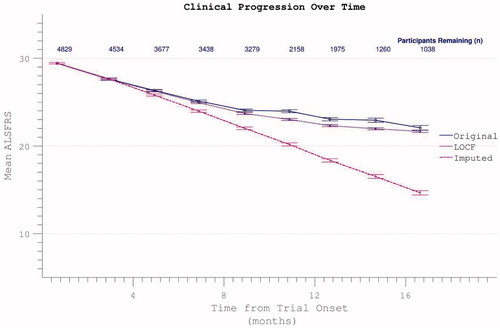
Figure 13. Confirmed participant death can be acknowledged by assigning zero values to subsequent ALSFRS scores, resulting in spurious apparent acceleration of disability progression. This effect is mitigated if other missing data (due to non-death trial withdrawal) are imputed by slope or carried forward (LOCF).