Figures & data
Figure 1. The effect of pH on catecholase (A) and cresolase (B) activities of entrapped polyphenol oxidase in alginate gel and alginate+κ-carrageenan polymer blends.
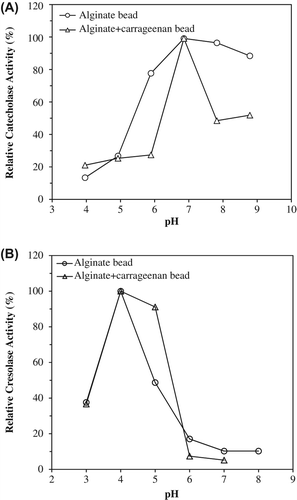
Figure 2. The effect of temperature on catecholase (A) and cresolase (B) activities entrapped polyphenol oxidase in alginate gel and alginate+κ-carrageenan polymer blends.
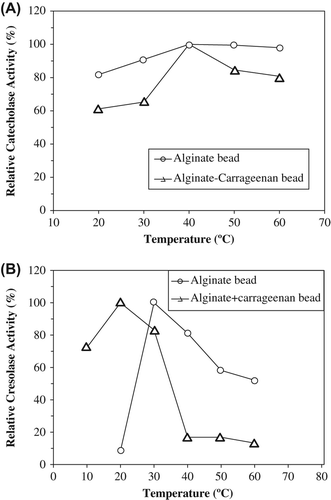
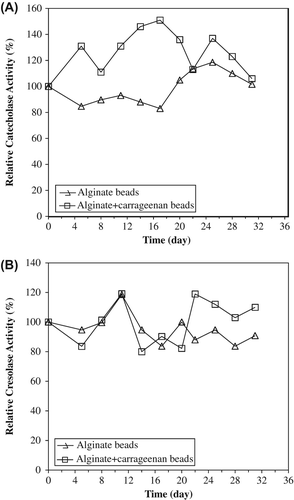
Figure 3. Kinetic parameters of entrapped polyphenol oxidase in alginate gel and alginate+κ-carrageenan polymer blends for catecholase (A) and cresolase (B) activities.
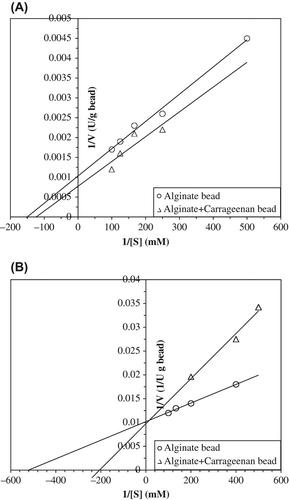
Table I. Km and Vmax values of entrapped artichoke polyphenol oxidase into alginate and alginate+κ-carrageenan gel beads for catecholase and cresolase activities.
Figure 4. The thermal stabilities of entrapped PPOs in alginate+κ-carrageenan beads (A) and alginate beads (B) for catecholase activities. The free enzyme and beads were incubated for various time intervals (15–60 min) at the specified temperature (50–60°C) and rapidly cooled. The activity was measured at 25°C, was taken as 100% and activities measured (50–60°C) were compared with the activity measured at 25°C. Empty symbols show immobilized enzyme (solid line), filled symbols free enzyme (dotted line). Each value is a mean of duplicate experiments.
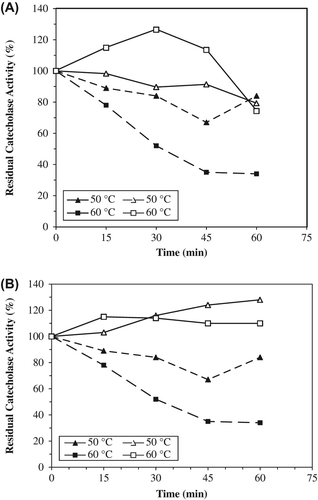
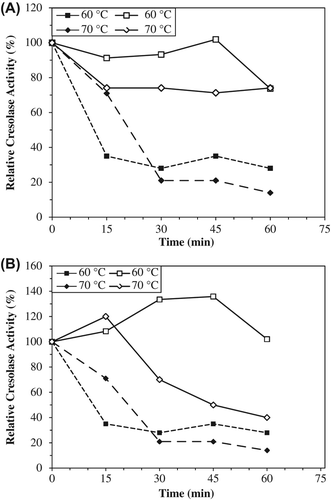
Figure 5. The thermal stabilities of entrapped PPOs in alginate+κ-carrageenan (A) and alginate gels (B) for cresolase activities. Empty symbols show immobilized enzyme (solid line), filled symbols free enzyme (dotted line). The free enzyme and beads were incubated for various time intervals (15–60 min) at the specified temperature (60–70°C) and rapidly cooled. The activity was measured at 25°C, was taken as 100% and activities measured (60–70°C) were compared with the activity measured at 25°C. Each value is a mean of duplicate experiments.