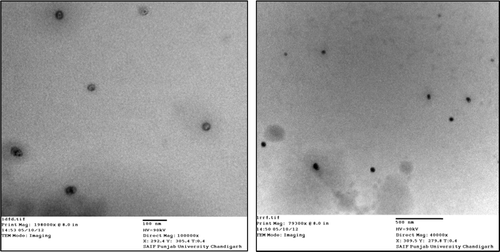
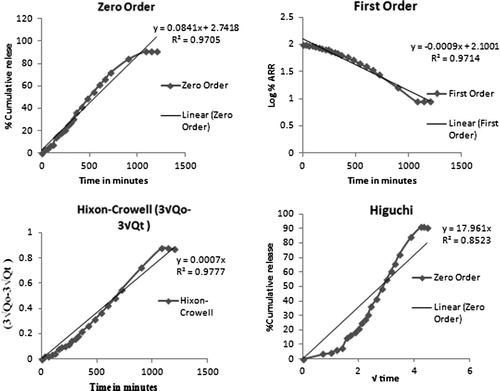
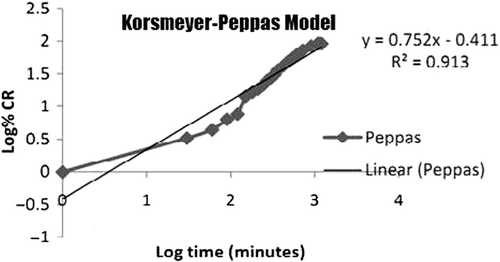
Figures & data
Table I. Selection of optimized concentration of oil.
Table II. Selection of optimized concentration of surfactant (n = 3).
Table III. Optimization of sonication time.
Table IV. Optimized composition for final formulation.
Table V. Optimized formulation along with their parameters (± S.D., n = 3).
Table VI. Stability studies of artemether nanoemulsion under accelerated stability conditions.