Figures & data
Table I. The five ratio-points of calibration standards weighed carefully for the four linear regression curves.a,b,c
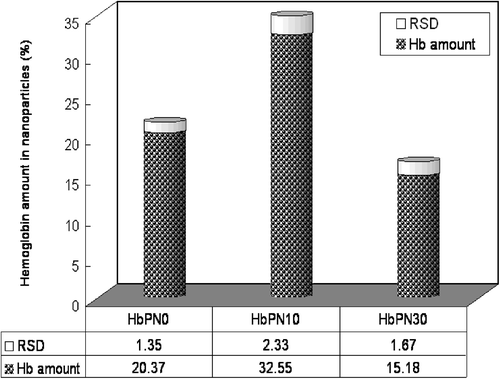
Table II. The actual weights of nanoparticles and PAN of each sample with the ratio be around 1:4.
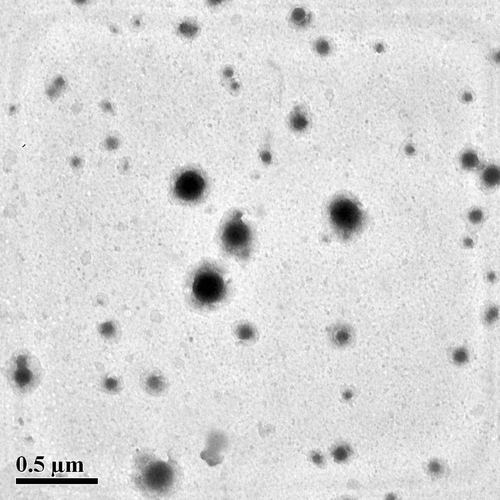
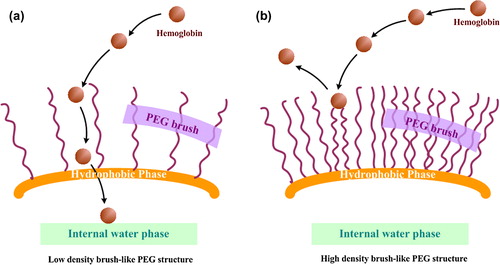
Table III. Particles size and surface charge of hemoglobin-loaded polymeric nanoparticles encapsulated by four types of polymer.
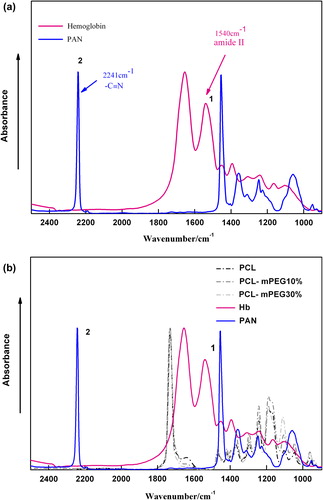
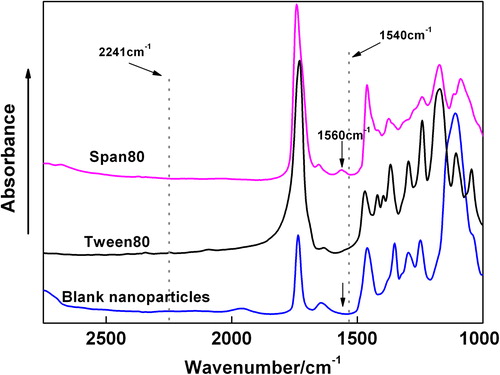
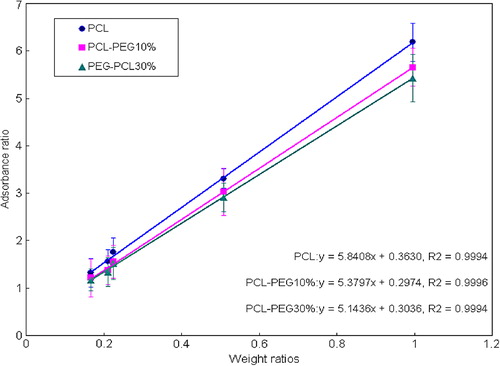
Table IV. Accuracy (percent recovery) of the method for the determination of Hb content in Hb-BPN mixture (n = 5).
Table V. The intra- and inter-day precision (relative standard deviation) of the method for the determination of Hb content in Hb-BPN mixture (percent of drug loading).