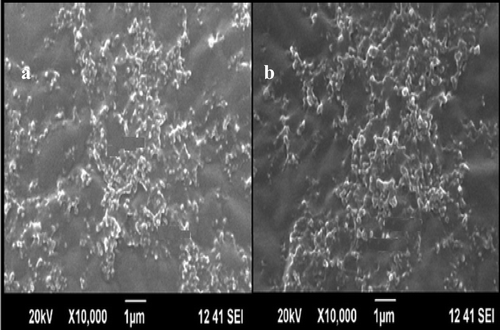
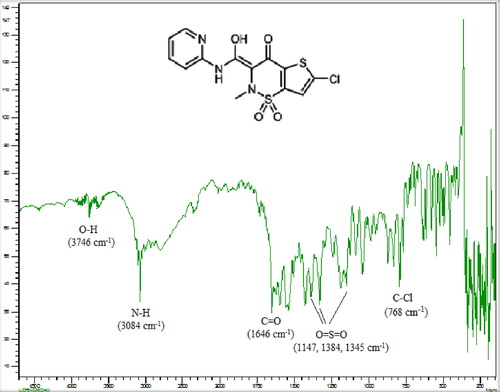
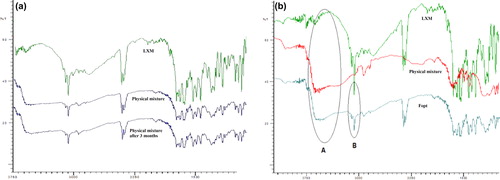
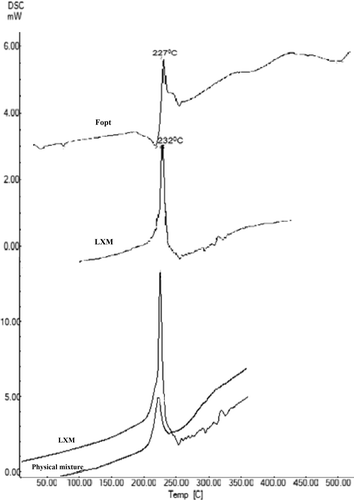
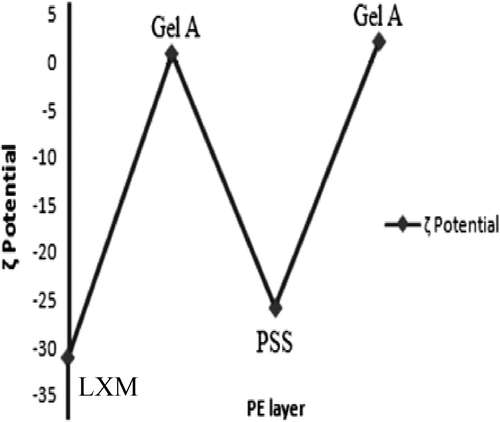
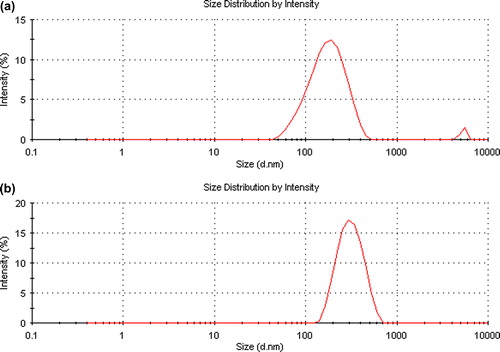
Figures & data
Table I. Layout of design actual.
Table II. Composition of factorial batches using 32 full factorial design.
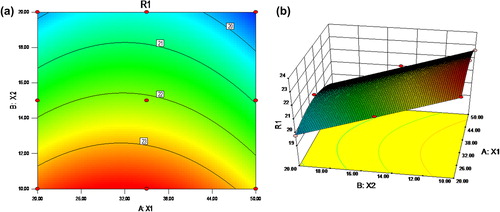
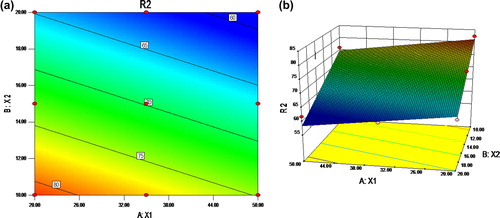
Table III. The ANOVA for percentage drug diffusion at 8 h.
Table IV. The ANOVA for percentage drug loading.
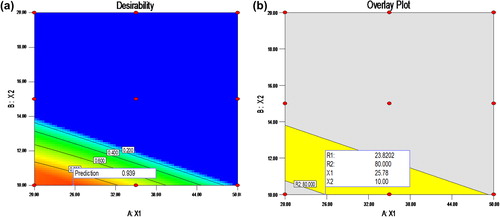
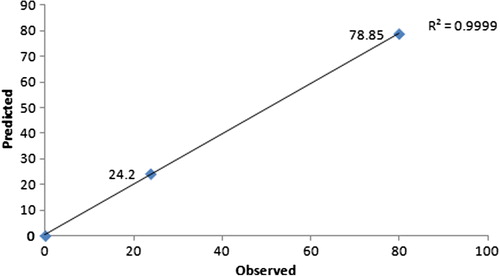
Table V. Predicted and observed value of response of Fopt and prediction error.