Figures & data
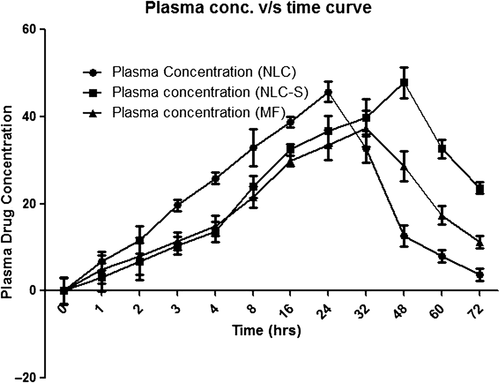
Table I. Experimental protocol for in vivo studies.
Table II. Optimization of homogenization time, sonication time, and ratio of solid lipid:liquid lipid.
Table III. Optimization of amount of atorvastatin calcium with respect to lipids ratio.
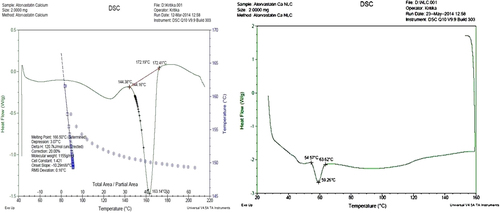
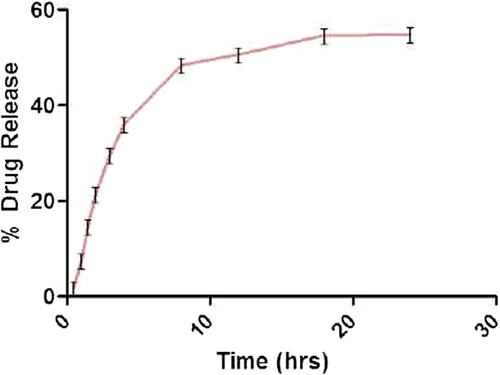
Table IV. Kinetics modeling of atorvastatin calcium NLC13.
Table V. Influence of spheronization speed on the size and shape of floating atorvastatin calcium spheroids.
Table VI. Influence of sizes of extruder die and spheronization plate on the properties of floating atorvastatin calcium spheroids.
Table VII. Optimization of HPMC-K4M with respect to lag floating time and floating time of spheroid.
Table VIII. Optimization of Carbopol 934 with respect to lag floating time and floating time of spheroid.
Table IX. Optimization of concentration of sodium bicarbonate of spheroid.
Table X. Optimization of concentration of citric acid of spheroid.
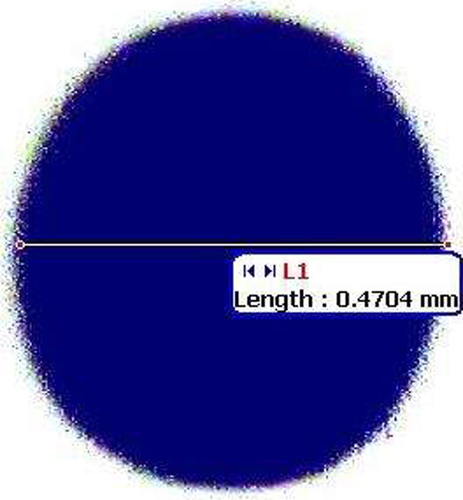
Table XI. Evaluation parameters for spheroids of atorvastatin calcium.
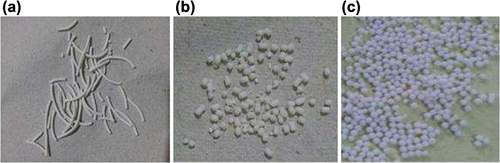
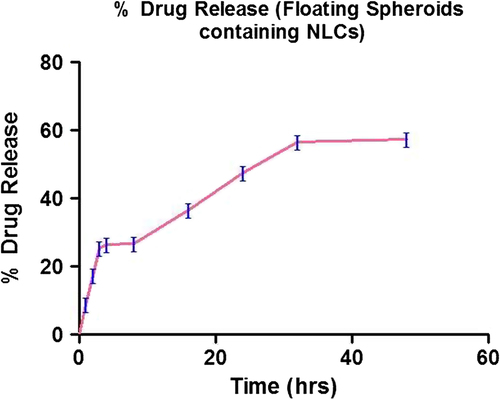
Table XII. In vitro release kinetic of floating atorvastatin calcium spheroids-loaded NLCs.