Figures & data
Figure 1. Drug-level analysis of OAD usage according PI recommendations. OAD, oral anti-diabetic drug; PI, prescribing information.
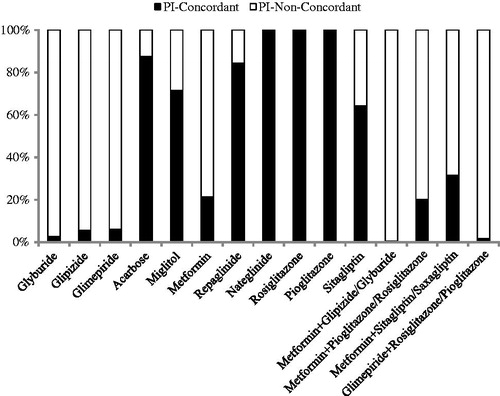
Table 1. Patient demographics, clinical characteristics, and healthcare costs and utilization at baseline evaluation period.
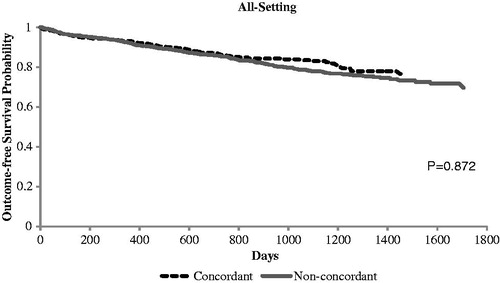
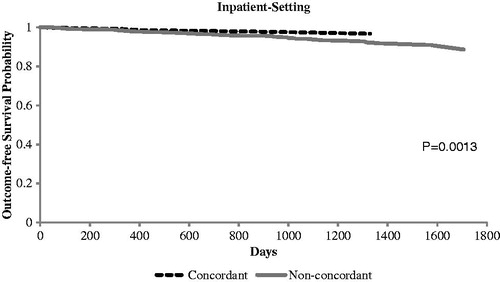
Table 2. Crude healthcare cost and clinical outcomes among PI-concordant and non-PI-concordant stages 3–5 CKD patients during 12-month follow-up period.
Table 3. Regression results for risk of severe hypoglycemic event.
Table 4. Regression results for glycemic control and annual inpatient admissions.
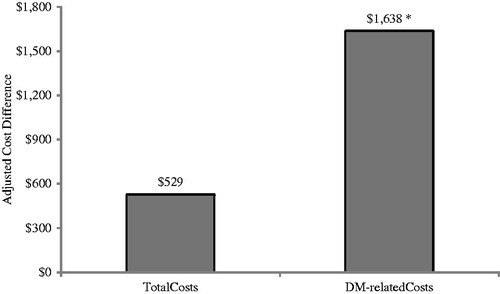
Figure 4. Adjusted 1-year cost differences associated with non-concordance to prescribing information. Model adjusted for age, gender, geographical region, CCI score, CKD stage, visits to specialists, insulin use, and any inpatient hospitalization during the baseline period. *Statistically significant at p < 0.05 compared to PI-concordant cohort.