Figures & data
Figure 1. Study Design. (A) Treatment-naïve: Patients were initiated on an ADHD medication for the first time on or after February 23, 2007, preceded by a 6-month period without ADHD drug prescriptions. The first drug on which a patient was initiated on or after February 23, 2007, and the date of its first fill date, based on pharmacy claims, were referred to as the index drug and the index date, respectively. (B) Previously treated: Patients were treated with an ADHD medication prior to February 23, 2007, and were switched to another medication on or after that date. Each new drug on which a patient was initiated on or after February 23, 2007, and the date of its first fill date, based on pharmacy claims, were referred to as the potential index drug and the potential index date, respectively. As patients may have initiated several ADHD medication over time, one index drug (and corresponding index date) was randomly selected among all potential index drugs (and corresponding potential index dates). The baseline period was defined as the 6-month period preceding the index date, while the study period was defined as the 12-month period following the index date. Treatment discontinuation was defined as an interruption of the index treatment for at least 30 consecutive days, that is, a gap between the end of one prescription and the start of the next prescription for the index drug of at least 30 days.
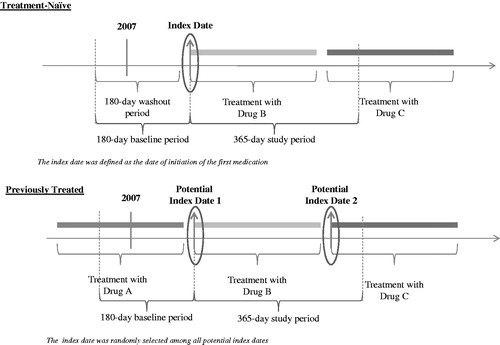
Table 1. Patients baseline demographic and clinical characteristics: Children and adolescents.
Table 2. Patients baseline demographic and clinical characteristics: Adults.
Table 3. Summary table: Comparison of adherence across all cohorts.
Table 4. Comparison of treatment adherence and probability of being adherent in patients treated with LDX vs other ADHD medications: Children and adolescents.
Table 5. Comparison of treatment adherence and probability of being adherent in patients treated with LDX vs other ADHD Medications: Adults.