Figures & data
Figure 1. Study design. * Patients who were already receiving oral aripiprazole treatment entered the open-label treatment phase (Phase B) without entering the oral conversion phase (Phase A). SOC, standard-of-care.
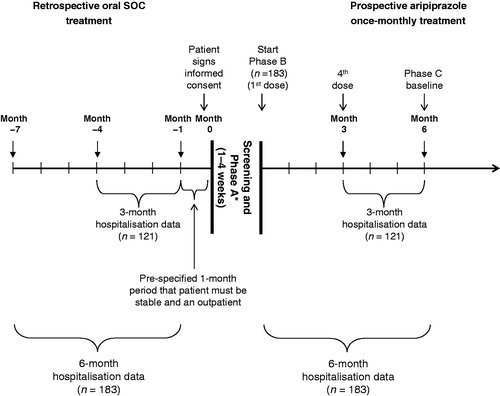
Table 1. Demographics and baseline characteristics for all patients that received treatment in Phase B (n = 181).
Table 2. Patient disposition and reasons for discontinuation.
Figure 2. Total psychiatric hospitalisation rates following the switch to aripiprazole once-monthly (prospective) compared with the same patients treated with oral anti-psychotics (retrospective). p-value derived from Exact McNemar test.
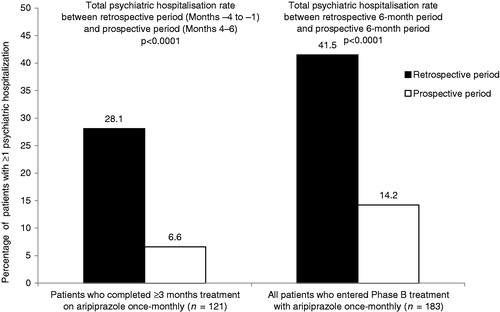
Table 3. Analysis of total psychiatric hospitalisation rates following the switch to aripiprazole once-monthly (prospective) compared with the same patients treated with oral anti-psychotics (retrospective) between retrospective period Months −4 to −1 and prospective period Months 4–6 and between retrospective period Months −7 to −1 and prospective period Months 1–6.
Table 4. Incidence of treatment-emergent adverse events occurring in ≥2% of all patients treated in Phase B (n = 181).