Figures & data
Figure 1. Treatment pathways. (A) B-R and CHOP-R. B-R: 28-day cycles; bendamustine 90 mg/m2/day on Days 1 and 2; rituximab 375 mg/m2 on Day 1; maximum six cycles. CHOP-R: 21-day cycles; cyclophosphamide 750 mg/m2 on Day 1; doxorubicin 50 mg/m2 on Day 1; vincristine 1.4 mg/m2 on Day 1; prednisone 100 mg/m2 on Days 1–5; rituximab 375 mg/m2 on Day 1; maximum six cycles. (B) CVP-R. CVP-R: 21-day cycles; cyclophosphamide 750 mg/m2 on Day 1; vincristine 1.4 mg/m2 on Day 1; prednisone 40 mg/m2 on Days 1–5; rituximab 375 mg/m2 on Day 1; maximum eight cycles. R-maintenance: 375 mg/m2 once every 8 weeks (first-line) and once every 3 months (second-line). Second-line treatment doses were taken from the approach by ScHARRCitation14. Early relapse, < 6 months after end of 24 months’ R-maintenance; late relapse, ≥6 months after end of 24 months’ R-maintenance. ASCT, autologous stem cell transplant; B, bendamustine; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; FC, fludarabine and cyclophosphamide; HDT, high-dose therapy; R, rituximab.
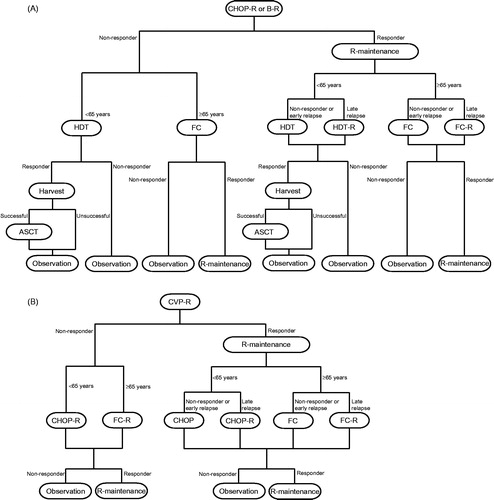
Table 1. Key clinical and quality-of-life inputs.
Table 2. Costs.
Table 3. Key results.
Table 4. Results of sensitivity analyses.
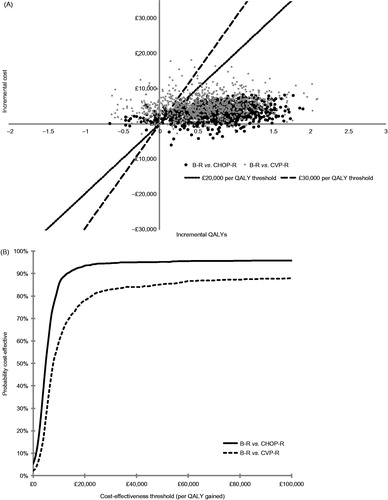
Figure 2. Results of probabilistic sensitivity analysis (1000 simulations). (A) Distribution of simulations on the cost-effectiveness plane. (B) Cost-effectiveness acceptability curve. B, bendamustine; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; QALY, quality-adjusted life year; R, rituximab.