Figures & data
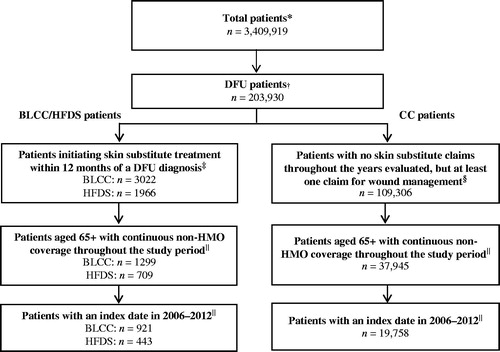
Figure 1. Selection of BLCC/HFDS and CC patients. BLCC: bioengineered living cellular construct; CC: conventional care; DFU: diabetic foot ulcer; HBO: hyperbaric oxygen; HFDS: human fibroblast-derived dermal substitute; HMO: health maintenance organization. *Total patient population includes all Medicare beneficiaries in the 5% Standard Analytic File with at least one medical claim in 2000–2012. †Includes patients with at least two distinct claims with a diabetes diagnosis (ICD-9-CM: 249.xx, 250.xx) and at least one claim with a foot ulcer diagnosis (ICD-9-CM: 707.10, 707.14 707.15, 707.19, 707.8, 707.9) on or after the first diabetes diagnosis at any time in 2000–2012 (the date of foot ulcer diagnosis was considered as the DFU diagnosis date). ‡Patients were required to have at least one claim for the specified skin substitute within 12 months following a DFU diagnosis (as defined above). Patients were not permitted to have a claim for the specified treatment during the 12 months preceding the treatment. §Patients were required to have no claims for skin substitutes at any time in 2000–2012 and were required to have at least one claim with either a DFU diagnosis, DFU-related procedure (debridement, drainage and incision, offloading, negative pressure wound therapy, HBO procedure), or a podiatrist visit. ||The study period was defined as the 12 months prior to and 18 months following the start of new treatment (index date) for the BLCC/HFDS patients, and following a random DFU-related claim (as defined above) meeting all sample selection criteria for the conventional care patients.