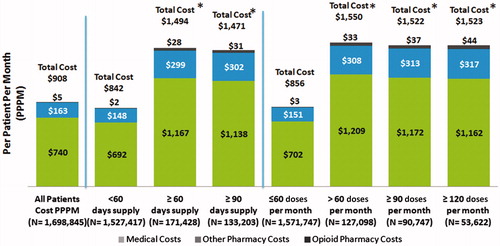
Figures & data
Figure 1. Sample selection. *Prescriptions for IR hydrocodone that were not pain-related (e.g., antitussive or sympathomimetic) were excluded.
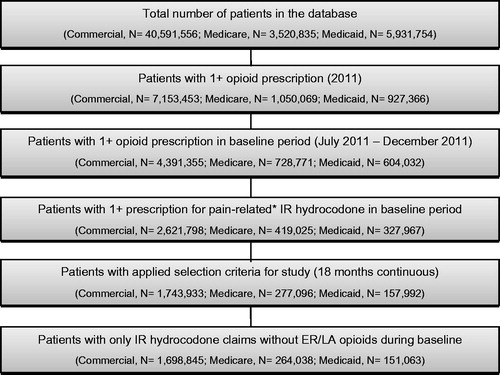
Table 1. Patient baseline characteristics.
Figure 2. Any HRU event during the 12-month follow-up period, by baseline days’ supply or doses per month of IR hydrocodone for commercial patients. *p-value is <0.05 vs <60 days’ supply or ≤60 doses/month.
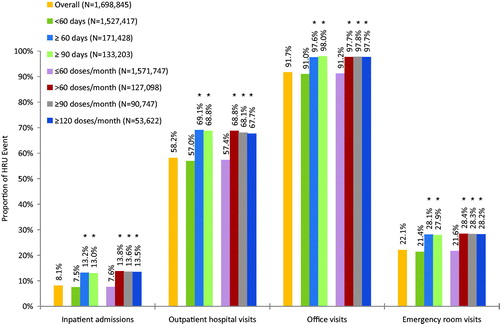
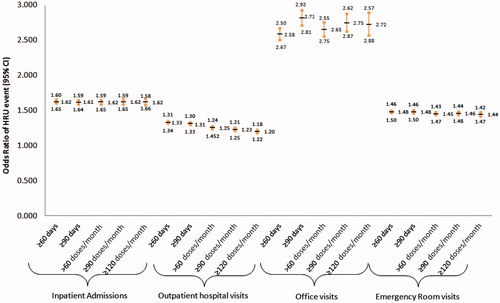
Figure 4. Odds ratio of HRU event by baseline days’ supply or doses per month of IR hydrocodone for commercial patients. All HRU events have p-value <0.05 for comparison against <60 days’ supply or ≤60 doses/month.