Figures & data
Fig. 1 Flow chart of the study participants.
Note. Several randomized participants were lost before baseline, most of them did not return their baseline symptom assessment and/or failed to find a time to meet the therapist in a timely manner. The intent to treat sample completed at least two symptom assessments and received at least one intervention session. Protocol violation in the control group entailed the following exclusion criteria : initiating trauma-related court litigation in one case and a loss of consciousness undiagnosed at the time of recruitment in two cases. Protocol violation in the intervention group entailed coming alone to the dyadic intervention and being diagnosed with a terminal illness during the study.
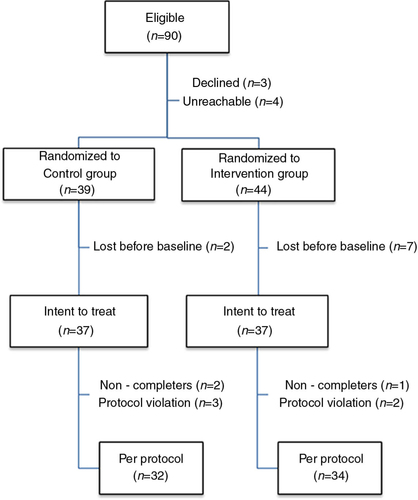
Table 1 Socio-demographic data: intent-to-treat population
Table 2 Brief and early dyadic intervention summary
Fig. 2 Self-reported mean PTSD symptom scores across time for the two study groups according the IES-R.
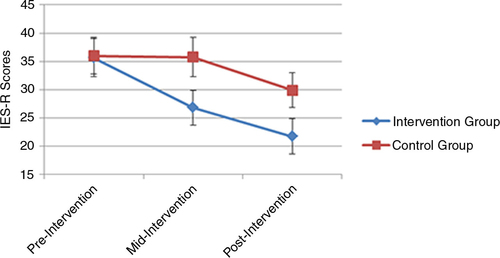
Table 3 Means and standard deviation of IES-R for every measurement time: intent-to-treat and per-protocol samples