Figures & data
Fig. 1. Transferrin (Tf) uptake and release by mesenchymal stem cells. a) Western blot analysis for the presence of CD71 (TfRs) in E1-MYC 16.3 cell lysate and HPLC-purified exosome preparations using anti-CD71; b) E1-MYC 16.3 cells were cultured in chamber slides. After incubating with 1, 5, 10 or 50 µg/mL of Alexa488-labelled Tf for 15 minutes, the cells were washed, fixed, counterstained with DAPI and observed by confocal microscopy. c) E1-MYC 16.3 cells were cultured in the presence of 1 µg/mL of Alexa488-labelled Tf for 30 minutes. The cultures were washed. Half of the cultures were fixed and counterstained with DAPI. The other half were cultured for another 30 minutes in the absence of the labelled Tf before they were similarly processed and observed by confocal microscopy; d) 105 E1-MYC 16.3 cells were plated into each well of a 6-well culture plate. After overnight incubation, cells were treated with 5 µg/mL of Tf-A488 (Life Technology, Grand Island, NY) and incubated for 15, 30, 60, 120 and 240 minutes. The cells were harvested and analysed by flow cytometry. e) 105 E1-MYC 16.3 cells were plated into each well of a 6-well culture plate. After overnight incubation, the cells in each well were serum-starved for 1 hour, exposed to 0, 7.5, 15 or 30 µm CPZ before washing with PBS and incubated for another 30 minutes with fresh serum-free medium containing 5 µg/mL of Tf-A488 and 0, 7.5, 15 or 30 µm CPZ. The cells were harvested and analysed by flow cytometry.
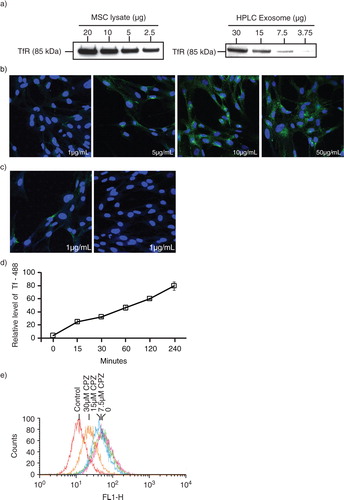
Fig. 2. Isolation of exosomes from transferrin (Tf)-fed MSCs. a) To assess uptake and release of biotinylated Tf, E1-MYC 16.3 cells were grown in chemically defined serum-free culture medium containing biotinylated Tf. The medium was harvested after 24, 48 and 72 hours of conditioning and purified by HPLC as described in Materials and Methods. The HPLC chromatogram illustrated the resolution of the components into 3 peaks in each of the conditioned medium. b) Western blot analysis of the 3 peaks in each conditioned medium for the presence of biotinylated Tf and CD9 using streptavidin-HRP and anti-CD9 antibody, respectively. Equal volume of each peak was loaded in each lane. c) TEM images of HPLC Peak 1, scale bar=200 ηm. d) 105 E1-MYC 16.3 cells were plated into each well of a 6-well culture plate. After overnight incubation, the cells were starved in chemically defined serum-free culture medium for 1 hour before exposure to 0, 7.5, 15 or 30 µM chlorpromazine (CPZ) for 30 minutes. The cells were washed with PBS and incubated for another 30 minutes with fresh serum-free medium containing 5 µg/mL of Tf-Biotin and 0, 7.5, 15 or 30 µM CPZ. The medium was then removed and replaced with fresh defined medium. After conditioning for 6 hours, the medium was collected and concentrated using an Amicon 100 kDa filter. The concentrated medium was immunoprecipitated with anti-CD81 antibody. The immunoprecipitate was analysed by western blot hybridization and probed for biotinylated Tf using streptavidin-conjugated HRP and CD9 using an anti-CD9 antibody. The signal ratio of the biotinylated Tf to CD9 is indicated for each concentration of CPZ.
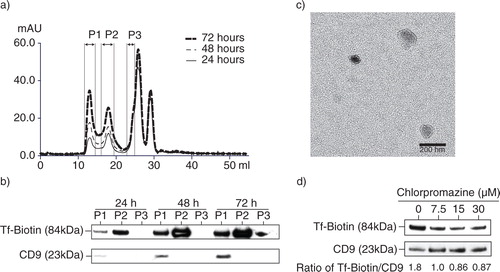
Fig. 3. Presence of CTB-binding domains in MSC exosomes. a) MSC exosomes were incubated with either biotinylated CTB or biotinylated CD81 antibody and extracted using streptavidin-conjugated beads. The beads were boiled in SDS-PAGE loading buffer and the proteins resolved by SDS-PAGE in a 4–12% gradient gel. After electroblotting the resolved proteins onto a nitrocellulose membrane, the proteins were probed for ALIX, TSG101 and CD9. b) 250 µg MSC CM was loaded on the top of a 22.8–60% sucrose density gradient and ultracentrifuged for 16.5 hours at 200,000×g, 4°C. After centrifugation, 13 fractions were collected starting from the top of the gradient. The density of each fraction was determined by weighing a fixed volume. Fifty microlitres of each fraction was then incubated with 0.5 ηg biotinylated CTB in 100 µL PBS pH7.4 to extract CTB-bound vesicles using streptavidin-conjugated magnetic beads. The bound vesicles on magnetic beads were then incubated with 100 µL 1: 500 diluted HRP-conjugated anti-CD81 antibodies to assay for the relative level of CD81. The level of CD81 was normalized to that in the lightest fraction, that is, 1.039 g/mL.
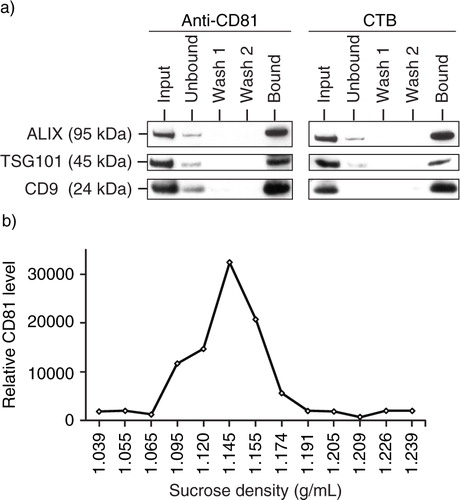
Fig. 4. GM1 gangliosides and TfRs are integral components of exosomes. a) After overnight incubation of 105 E1-MYC 16.3 cells in each well of a 6-well culture plate, the cells were treated with 5 µg/mL of CTB-488 and incubated for 15, 30, 60, 120 and 240 minutes before flow cytometry analysis; b) after overnight incubation of 3×106 E1-MYC 16.3 cells in a 10-cm tissue culture dish, the cells were washed and incubated in medium containing 5 µg/mL biotinylated CTB for 30 minutes before washing and replacing culture with fresh medium. At 0.5, 1, 2, 4, 8, 16 and 32 hours, the medium was harvested and replaced with fresh medium. The harvested CM was concentrated and assayed for CTB-coupled CD81 by ELSA; c) To confirm the co-localization of GM1 gangliosides and TfRs in exosomes, HPLC-exosome fraction was extracted with excess CTB or Tf. Relative levels of CTB- and Tf-binding vesicles were determined by measuring CD81. The extracted fractions were further extracted with Tf or CTB, respectively. These second extracts were also assayed for CD81.
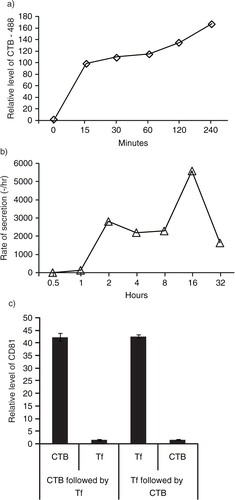
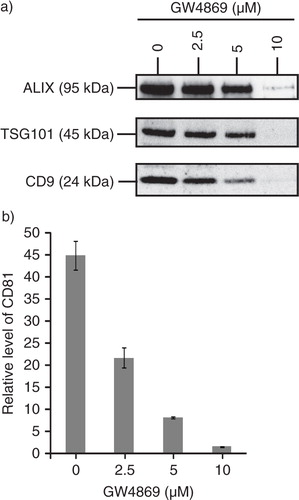
Fig. 5. MSC exosome production is dependent on sphingomyelinase. 105 E1-MYC 16.3 cells were plated into each well of a 6-well culture plate, treated with 0, 2.5, 5 or 10 µM GW4869 (nSMases Inhibitor) for 1 hour, washed and incubated overnight. The conditioned medium was harvested and concentrated. The concentrated medium was analysed by a) western blot (25 µg); and b) CTB-coupled CD81 ELISA assay (10 µg).