Figures & data
Fig. 1. Extracellular vesicle (EV) origin: EV may originate from the endosomal compartment by exocytosis of vesicles formed within the multivesicular bodies or (a) from the cell surface by budding of plasma membrane. These shedding vesicles, sorted from the cell surface by budding of cell plasma membrane, are also named microvesicles. (b) Exocytic multivesicular bodies fuse with membrane after cell stimulation and release by exocytosis vesicles named exosomes. (b) These multivesicular bodies are created within the Golgi apparatus as a result of endosome compartmentalization. The insets are representative transmission electron microscopy of exosome generation from a multivesicular body and of vesicle generation by budding of plasma membrane (modified, in part, from Refs. (Citation3) and 7).
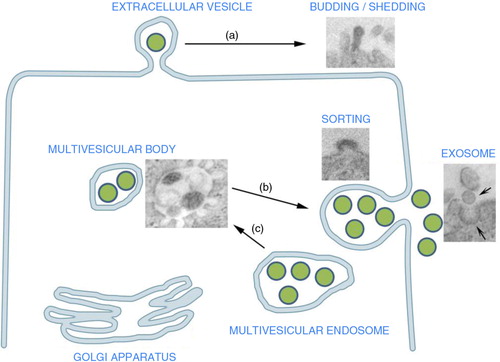
Table I. Extracellular vesicle mechanisms of action with respect to fertility
Fig. 2. Tumour-derived EV and local invasion and metastasis: EV derived from primary tumour act to enhance matrix remodelling via a) matrix metalloproteinase (MMP) and urokinase-type plasminogen activator (uPA), enhance b) endothelial angiogenesis via vascular endothelial growth factor (VEGF) and c) EV released from PC3 cells activate fibroblasts sending antiapoptotic signals and growth signals (Citation23, Citation25). Ultimately, EV tumour release leads to downstream enhanced tumour cell migration, adhesion and invasion.
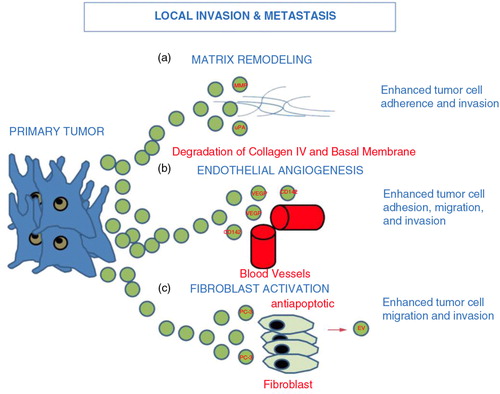
Fig. 3. Tumour cells release extracellular vesicles that can influence the malignant phenotype. Various examples include and are not limited to: (a) drug resistance; EV can influence the efflux of chemotherapeutic drugs via various mechanisms including ATPase and drug transporters. (b) apoptosis; through FasL and TRAIL, EV can induce apoptosis in activated antitumor T cells, abrogating T-cell-mediated apoptosis of tumour cells. (c) Local invasion and metastasis; EV can promote local invasion by activating fibroblasts and reducing fibroblast apoptosis, enhancing extracellular matrix degradation (mRNAs for MMP2 and MMP9), promoting angiogenesis (mRNA VEGF, FGF2, angiopoietin1) and increasing tumour cell adhesion. EV may enhance metastasis by promoting a pre-metastatic niche in lung tissue via upregulation of VEGFR1 expression, MMP2 in lung blood vessels and MMP9 in alveolar epithelial cells and blood vessels. (d) Immunosuppression; EV can alter monocyte differentiation into myeloid suppressive cells. This inhibits T-cell proliferation. Inhibiting T-cell responses upstream would abrogate antitumor immune potential. This figure is modified from (Citation66).
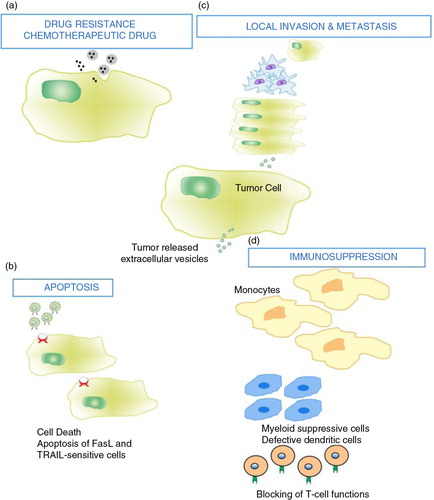
Fig. 4. Tumour extracellular vesicles and chemoresistance (Citation67). Tumour EV act in 2 ways to reduce the efficacy of chemotherapy. (Citation1) intracytoplasmic chemotherapy exportation via shedding vesicles. (Citation2) Antibody sequestration.
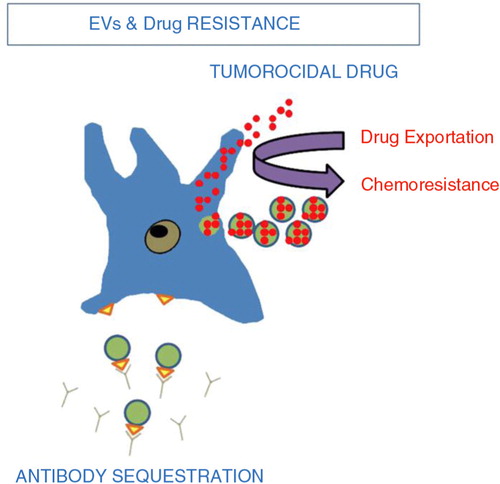
Table II. Proposed therapeutic strategies to mitigate extracellular vesicle effects in carcinogenesis
Table III. Extracellular vesicle characteristics that are favourable as a vehicle of therapy
