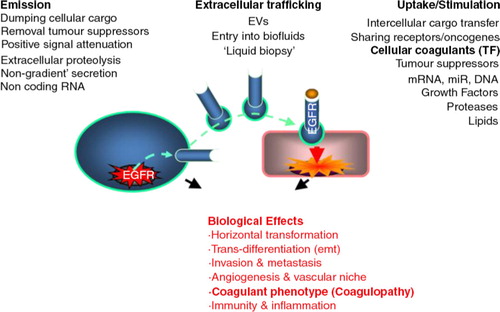
Figures & data
Fig. 2. Tissue factor initiates coagulation by binding factor VIIa to form membrane bound complex which activates factor X. This forms a complex with factor Va which activates prothrombin to thrombin. Thrombin activates factor XI which creates an amplification loop through the factor IXa/VIIIa activation of factor X. The resulting burst of thrombin generation causes platelet activation and conversion of insoluble fibrinogen to an insoluble thrombin clot.
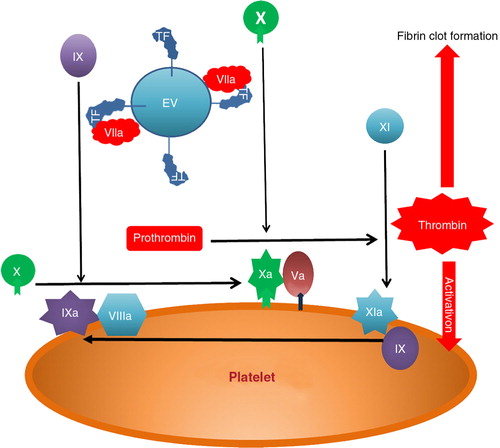
Fig. 3. (a) TF-bearing EVs in glioma and meningioma patients and in healthy controls. (b) Origin of TF-bearing EVs in glioma patients and controls.
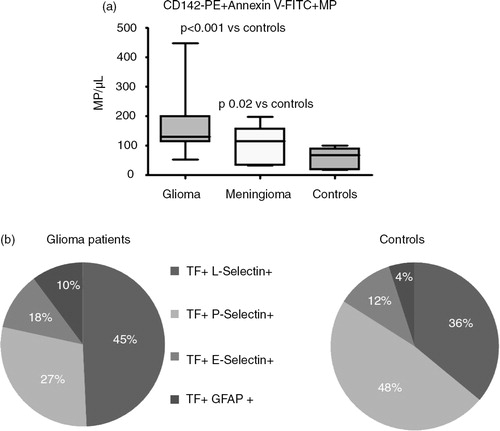
Fig. 4. (a) The phosphorylation of Ser253 within the cytoplasmic domain acts to initiate the incorporation and release of TF within EVs. (b) The interaction between TF and filamin-A is required for the active incorporation of TF into EVs.
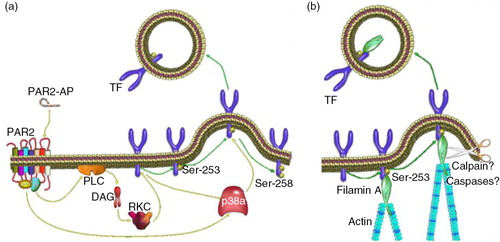
Fig. 5. Model for the release of TF-exposing vesicles. TF-exposing vesicles originating from different membrane compartments.
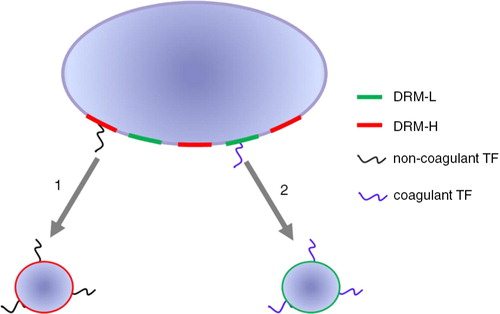
Fig. 6. TF-dependent coagulation activation is of relevance for metastasis, tumour growth, and angiogenesis through several distinct mechanisms, including the activation of thrombin further resulting in the generation of a fibrin network, and platelet activation. These end stages of coagulation can contribute to tumour progression and metastasis by, for example, protection of circulating cancer cells from immune cell attack. In addition, TF and associated coagulation proteases up-stream of thrombin can cleave and activate protease-activated receptors (PARs) to induce pro-migratory and survival signalling in cancer cells. Endothelial cells, however, are normally devoid in TF. At hypoxic conditions, cancer cells may release TF/FVIIa-bearing EVs that in a paracrine manner trigger an angiogenic response through activation of PAR-2 in endothelial cells. Further, it may be hypothesized that systemic release of TF-EVs from hypoxic tumour regions contributes to the hypercoagulable state of cancer patients.
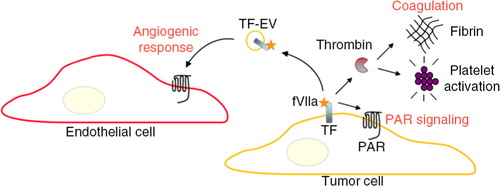
Table I. Issues with measurement of MV-associated TF.