Figures & data
Fig. 1. Vastly diverse vesicles and other membrane compartments found in human ejaculate. (a) A cryo-electron micrograph of human sperm tail surrounded by small, electron dense particles (black arrows) as well as EVs (white arrows). (b) A cryo-electron micrograph of a sperm tail, EVs (white arrows) and other membrane compartments (white arrowheads). (c) The size distribution of all particles and membranous compartments in human ejaculates. (d) Prevalence of particles and all membranous compartments in the seminal fluid. (e) The size distribution of EVs and membranous compartments in human ejaculate from all 7 ejaculates pooled (excluding particles lacking lipid bilayer; number in brackets represents the amount of vesicles with a larger diameter than displayed in this graph). (f) The prevalence of each EV or membrane compartment. Clockwise: single vesicles, oval vesicle, double vesicle, double special vesicle, triple to 6 vesicles, incomplete vesicles, small tubules, large tubules, pleomorphic membrane structures, vesicle sacs and lamellar bodies. (g) Sizes of vesicles/membrane compartments in each subcategory (average and standard deviation). (h) A 10-nm-thick slice from a tomographic reconstruction of a double special vesicle. (i) 3D model of the vesicle shown in h.
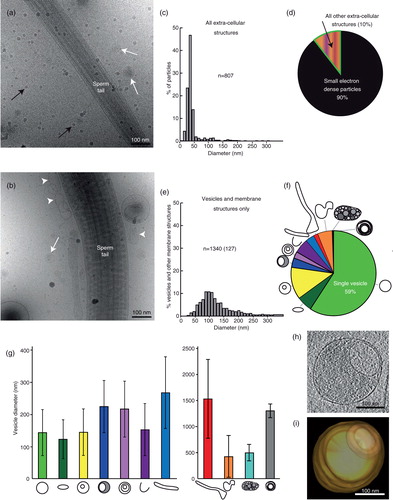
Fig. 2. Gallery of extracellular vesicle subcategories. (a–e) Three cryo-electron micrographs are shown for each category, followed by the EV size distribution of that subcategory. n=sample size (amount of vesicles above 500 nm in diameter). Scale bars=50 nm.
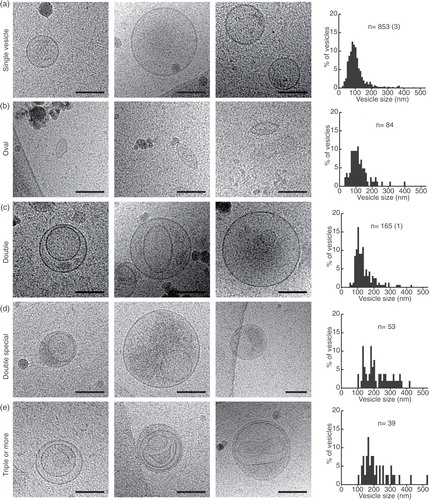
Fig. 3. Gallery of different membrane compartments. (a) Incomplete vesicles and their size distribution. (b) Thin tubules and their size distributions. (c) Large tubule, captured over several overlapping images and line drawings to show their complicated internal relations. (d) A large tubule containing filaments (probably actin) and a line drawing showing the directionality of the filaments. Scale bars=50 nm.
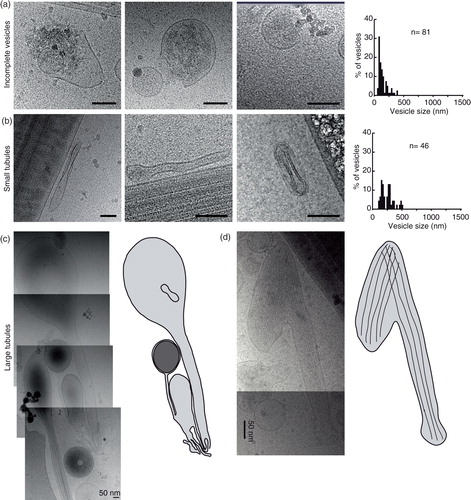
Fig. 4. Pleomorphic membrane structures – a diverse group of membrane compartments. (a) A large membrane-bound structure in close apposition to a sperm tail. (b) A large membrane compartment. (c) A tubular membrane structure containing a larger vesicle containing 2 smaller vesicles. (d) A figure 8-shaped membrane compartment containing a smaller oval membrane compartment. (e) Size distributions of pleomorphic membrane compartments. Scale bars=50 nm.
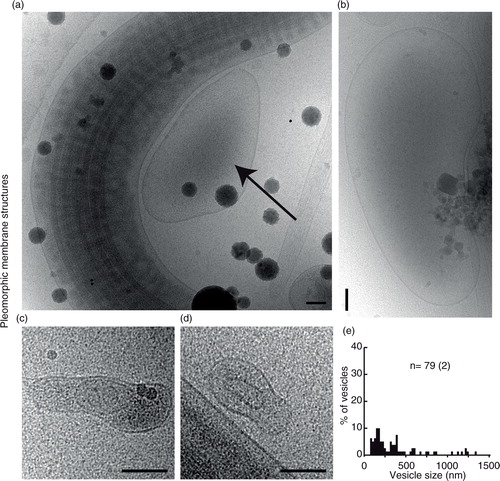
Fig. 5. Vesicle sacs and lamellar bodies. (a) Two overlapping cryo-electron micrographs showing a large collection of vesicles inside a membrane (left) and a similar ruptured structure (right). (b) A line drawing of the vesicles and membranes surrounding them in (a). Note that vesicles of different categories are present in the same vesicle sac (turquoise). (c) An example of a smaller, intact, vesicle sac. (d) A lamellar body showing multilayered membranes that are not always closed, shown as a line drawing in (e).
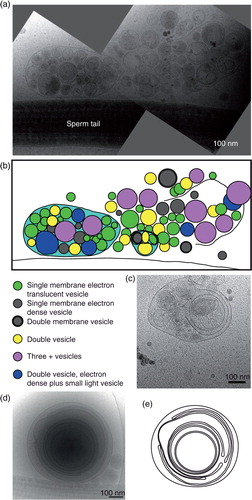
Fig. 6. Three special features can occur in any of the 11 subcategories. (a) Prevalence of the different features in the whole EV and membrane compartment population. (b and c) Membrane compartments with a coated membrane. (d) A logarithmic graph displaying the vesicle coat height as a correlation to the EV/compartment size. Colour coding shows that EVs of many categories, as well as membrane compartments, can be coated. (e) Three examples of electron dense vesicles. (f) The size distribution of electron dense vesicles. (g) Three examples of vesicles with double membrane bilayers. (h) The size distribution of all double bilayered EVs/compartments.
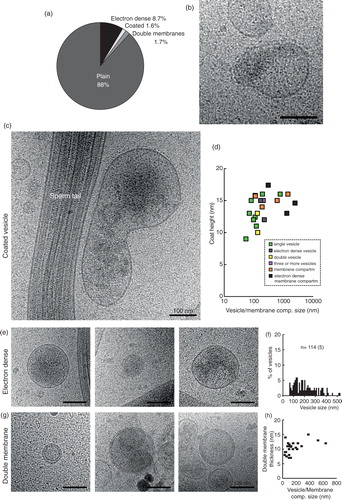
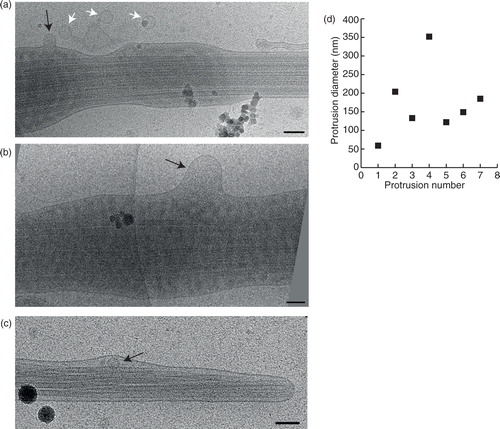
Fig. 7. Fusion or budding of vesicles to/from the sperm tail. (a) A small vesicle (black arrow) fusing into, or budding from the sperm tail. Three more vesicles of a similar size appear to be incoming/outgoing from this budding/fusion site. (b) A larger membrane protrusion (black arrow) looks like a budding/fusion event at the sperm tail. (c) Membrane deformation around this small vesicle (black arrow) shows that it is located inside the sperm end piece. (d) Measurements of all the membrane protrusions seen. Scale bars=100 nm.