Figures & data
Fig. 2. Organisation of the French HTA and pricing system. Dashed arrows indicate the support of the subcommittees/services to the different committees in their work.
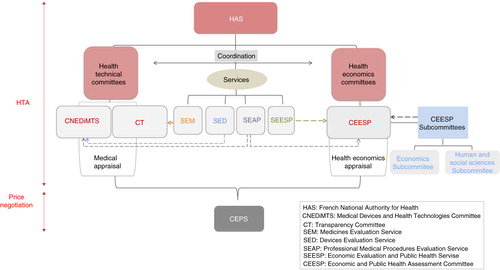
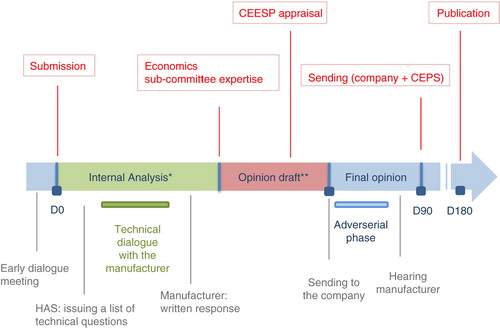
Fig. 3. The coordinated assessment/appraisal (Citation8).
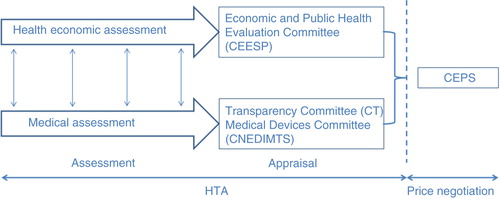
Fig. 5. Economic appraisal process. Adapted from HAS, Economic appraisal process (Citation8). *Administrative compliance and scientific/methodological compliance. Key actors: 2–3 project managers from the Health Economics and Public Health Department (SEESP) + economics sub-committee rapporteur + possibility to submit questions to external clinical and/or methodological experts. **Key actors: the CEESP members: economic, clinical public health, and social science experts (monthly meeting).