Figures & data
Fig. 1. Behavioral videos of propionic acid infusions in rats (click headings to view videos). Single intracerebroventricular (ICV) infusions (4 µl of 0.26 M solution over 4 min) of propionic acid (PPA), a metabolic end product of autism-associated enteric bacteria, produce bouts of reversible hyperactive and repetitive behavior (A) in adult rats, compared with phosphate-buffered saline (PBS) vehicle infused control rat (B). Rat pairs infused with PPA show markedly reduced social interaction and play behavior (C), compared with pairs of rats infused with PBS vehicle (D), which show typical social behavior. Ethovision behavioral tracking of control and PPA-treated rat pairs (E), showing further evidence of PPA-induced hyperactive, repetitive and antisocial behavior. PPA-treated rat displays fixation on objects (F) and a specific object preferences (i.e. block vs. sphere). PPA-infused rats also show turning, tics, dystonia, and retropulsion and electrographic evidence of complex partial seizures and basal ganglial spiking, consistent with findings in patients with autism spectrum disorders.
A – PPA repetitive behavior
B – control rat
C – PPA social
D – control pair social
E – Ethovision pair
F –PPA object fixation

Fig. 2. (A) Intracebroventricular (ICV) infusions of propionic acid (PPA) in adult rats increase repetitive locomotor activity (Versamax automated behavioral assay). Group mean values (±SEM) total distance, number of movements, and movement time in rats given 4 µl ICV infusions of phosphate-buffered saline (PBS) vehicle or PPA (0.26 M) twice daily for 7 days. BL = baseline session with no infusion; T1–T7 consecutive treatment days. *p<0.05. (B) Single ICV infusions (4 µl of 0.26 M solution over min) of PPA and isomolar acetic acid (SA) in adult rat pairs reduce social behavior (Ethovision automated behavioral assay). Behavior is measured as mean distance apart (cm) and time spent (%) in 5 cm proximity during dark hours. Data points represent group means of data collected during 10 min periods. Animals were placed in a large open field in same-drug pairs. Both PPA and SA pairs displayed a significantly greater mean distance apart and spent significantly less time in close proximity to each other, consistent with impaired social behavior found in autism, compared with phosphate-buffered saline (PBS) vehicle controls. Propanol the non-acidic alcohol analogue of PPA was without effect. *=different from PBS control group at p<0.05 or better. (C) Tracks representing object and socially related behavioral movement of adolescent rats receiving ICV infusions of PPA (4 µl of 0.26 M solution over 4 min) or PBS. More locomotion near the caged novel rat (NR) occurred in PBS rats than by PPA rats. Graphic representation of duration of time (s) spent in close proximity (within 18 cm) of the novel rat or novel object (NO). PPA rats showed less approach behavior and remain close to the novel rat less than PBS rats, indicative of object preference over social behavior, consistent with findings in patients with autism. Graphic representation of group mean (±SEM) percent correct turns in the T-maze task during acquisition (Days 1 and 2) and reversal (Days 3 and 4). + = significant difference from Day 2 performance; * = significant different from PBS control group. Figures modified with permission from MacFabe et al. (2008) and (2011), and Shultz et al. (2008). A reproduced with permission from Science (American Association for the Advancement of Science). B and reproduced with permission from Elsevier Ltd.
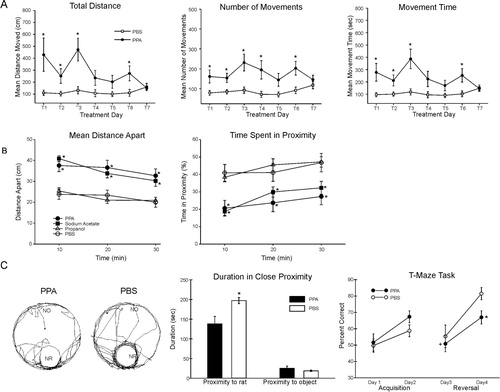
Fig. 3. (I) Intracerebroventricular (ICV) propionic acid (PPA) infusions in rats induce electrographic and behavioral elicitation of kindled seizures and movement disorder. Kindled seizure manifestation in response to repeated weekly 4 µl ICV infusions of low (0.052 M) or high (0.26 M) PPA, isomolar acetic acid (SA), propanol, or phosphate-buffered saline vehicle (PBS) in adult rats: group mean (±SEM) data summed across the five initial testing sessions. Latency to epileptiform spiking was measured from the end of the ICV infusion to the start of seizure. Maximum convulsion stage was rated using the Racine kindling scale. Duration of the longest convulsion was of the longest continuous convulsion during a session. PPA and, to a lesser extent, SA induce a kindling response, while propanol, the non-acidic analogue of PPA, was without effect. * = different from propanol and PBS controls; # = different from low PPA, propanol control, and PBS control; + = different from propanol control; ** = different from all other groups. (II) Abnormal behaviors in response to ICV infusions: group mean frequency (±SEM) of behaviors per baseline or initial test session. Either one or both doses of PPA increased the abnormal behaviors relative to control treatments. Only PPA produced dystonic (snake like), retropulsive behaviors. With the exception of sodium acetate, which increased turning, no control treatments increased abnormal behavior. Black bars indicate treated animals; white bars indicate PBS (vehicle)-treated animals. * = p < 0.05 or better vs. all control groups; # = p < 0.05 or better vs. low PPA, propanol, and PBS control. (III) Representative electrographic seizure records from rat in the high PPA group. (A) Session 2, short bout of epileptiform spiking accompanied by contralateral hindlimb dystonia coincident with spiking (event marker). Note spiking in frontal cortex and caudate but not dorsal hippocampus. (B) Session 3, single epileptiform spikes in caudate and frontal cortex but not hippocampus. Only the caudate spikes were accompanied by brief contralateral hindlimb dystonia (event markers), which led the frontal cortex spikes by approximately 500 ms. A prominent frontal cortex spike (arrow) is not accompanied by a spike in the caudate or by limb dystonia. (C) Session 5, bout of spiking in dorsal hippocampus not accompanied by corresponding bouts of spiking in frontal cortex or caudate or by limb dystonia. (D) Session 5, single epileptiform spikes occur first in the caudate and lead spikes in other traces. These are followed by a short bout of epileptiform spiking that is accompanied by brief retropulsion, followed immediately by contralateral hindlimb dystonia, which ends coincident with the end of the bout of spiking (event marker). (E) Session 5 at approximately 1 min after the records in D, single epileptiform spikes in all three traces, with the caudate spikes leading the spikes in the other traces. Each spike is accompanied by brief contralateral hindlimb adduction and immediate dystonia (event markers). (F) Session 5 at approximately 6 min after the records in E, short bout of epileptiform spiking with hindlimb dystonia beginning coincident with caudate spiking (event marker), with frontal cortex and hippocampal spiking beginning after onset of caudate spiking and hindlimb dystonia. This is followed 2 s later by the beginning of the first sustained kindled seizure displayed by this rat (duration = 35s), which was accompanied by the first conventional kindled convulsion (Stage 2), which did not include limb dystonia. C = caudate; FC = frontal cortex; H = dorsal hippocampus; HD = hindlimb dystonia. Amplitude calibration = 50 µv; time marker = 5 s. Figures modified with permission from MacFabe et al. (2007). Reproduced with permission from Elsevier Ltd.
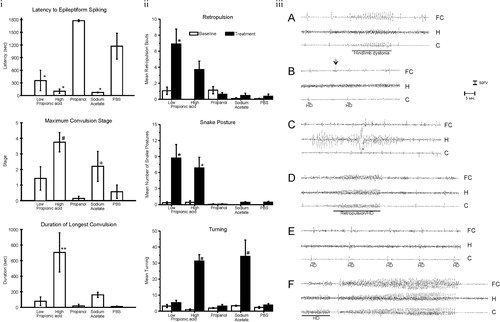
Fig. 4. (A) Propionic acid (PPA) infusions in rats induces a significant increase in lipid and protein oxidation, supportive of increased oxidative stress via increased oxidant production and decreased antioxidant defenses in homogenates of discrete regions of rodent brain consistent with findings in patients with autism. Interestingly, brain stem appears relatively unaffected. (B) PPA-treated rats showed significantly decreased total GSH, a decreased activity of GPx, but an increase in the activity of GST. Conversely, the activity of GR was unchanged. These findings suggest that GSH may be involved in the metabolic clearance of PPA, or alternatively, the synthesis of GSH was impaired by PPA. The observed reduction of the activity of GPx may be due to the decreased availability of GSH, a cofactor of GPx or may reflect an overall reduction in antioxidant defenses induced by PPA. Black bars indicate PPA-treated animals; white bars indicate PBS (vehicle)-treated animals. Figures modified with permission from MacFabe et al. (2008). Reproduced with permission from Science (American Association for the Advancement of Science).
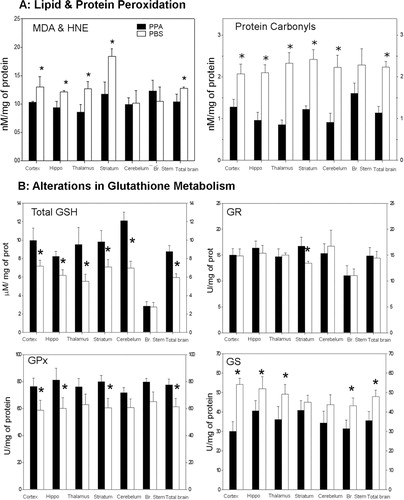
Fig. 5. Neuropathology (avidin–biotin complex immunohistochemistry) and semiquantitative image densitometry of coronal brain sections of dorsal hippocampus (CA2) and external capsule of adult rats with 14 day BID ICV infusions of Propionic acid (PPA) or phosphate-buffered saline (PBS). PPA induced significant reactive astrogliosis (anti-GFAP) and microglial activation (anti-CD68), without apoptotic neuronal cell loss (anit-cleaved caspase 3) in rat hippocampus, similar to finding in autopsy brain from patients with autism. Nuclear translocation of anti-CREB and an increase of anti phosphoCREB immunoreactivity are observed in neural, glial, and endovascular epithelium by PPA treatment, suggestive of gene induction. PPA increases monocarboxylate transporter 1 immunoreactivity, primarily in white matter external capsule, suggestive of alterations in brain shot-chain fatty acid transport/metabolism. Black bars indicate PPA-treated animals; white bars indicate PBS (vehicle)-treated animals. Horizontal measurement bar = 100µ. Figures modified with permission from MacFabe et al. (2007); see publication for details of immunostaining procedures. Reproduced with permission from Elsevier Ltd.
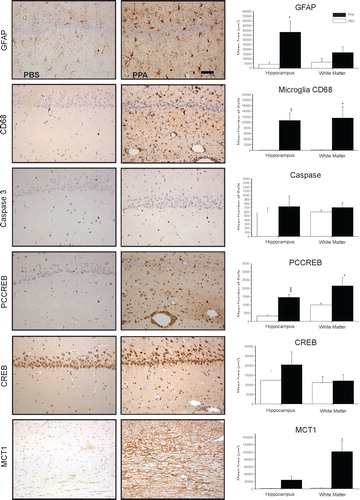
Fig. 6. (A) Changes in phosphatidylethanolamine and phosphatidylcholine in rat brain tissue following repeated 4 µl intracerebroventricular infusions with phosphate-buffered buffer (PBS), butyric acid (BUT), and propionic acid (PPA) 0.26 M twice daily for 7 days are consistent with findings in patients with autism. Values represent means±SE. Arrows indicate significant difference between treatments [PPA and BUT compared with the control (PBS)], and direction of the changes (increase or decrease) at LSD = 0.05, N=6 per treatment. Monounsat = monosaturates; polyunsat = polyunsaturates. (B) Changes in acylcarnitine and ratio of bound to free carnitine in rat brain tissue following intracerebroventricular infusion with PBS, BUT, and PPA are consistent with findings in patients with autism. Values represent means±SE. Means accompanied by different superscript letters (e.g. a, b, and c) indicate significant difference between treatments at LSD = 0.05, N=6 per treatment. Long-chain acylcarnitine = C12–C24, short-chain acylcarnitine = C2–C9. (C) PPA and, to a lesser extent, BUT infusions increase short- and long-chain acylcarnitines, but not medium-chain acylcarnitines, similar to findings in patients with autism. Values (percent by weight) represent means±SE. Means in the same row accompanied by different superscript letters (e.g. a, b, and c) are significantly different between treatments at LSD = 0.05, N=9. Numbers preceding C represents carbon number of the fatty acid moiety attach to carnitine. Long-chain acylcarnitine = C12–C24, short-chain acylcarnitine = C2–C9. Figures modified with permission from Thomas et al. (2010). Reproduced with permission from Wiley Blackwell.
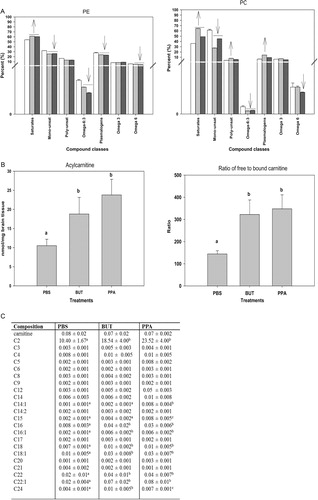
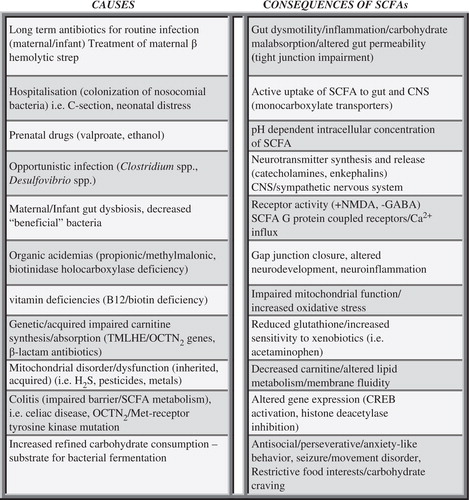
Fig. 7. Potential causes and consequences of increased enteric short-chain fatty acid production and/or decreased breakdown and their relation to autism spectrum disorder. These findings, which are not mutually exclusive, may contribute to the pathophysiology, behavioral symptoms, and comorbidities of autism.