Figures & data
Fig. 2 Typical examples of 5-d backward air mass trajectories arriving at Morogoro during May to August 2011 sampling period.
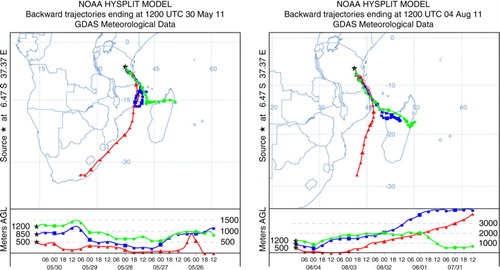
Table 1. Stable carbon isotope ratios (δ13C, ‰) of dicarboxylic acids, glyoxylic acid and glyoxal in PM2.5 aerosols collected from Tanzania, in July and August 2011
Table 2. Stable carbon isotope ratios (δ13C, ‰) of dicarboxylic acids, glyoxylic acid and glyoxal in PM10 aerosols collected from Tanzania, in May through August 2011
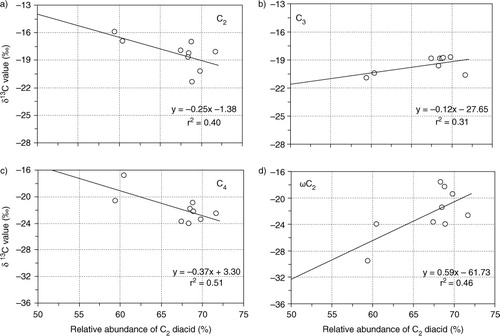
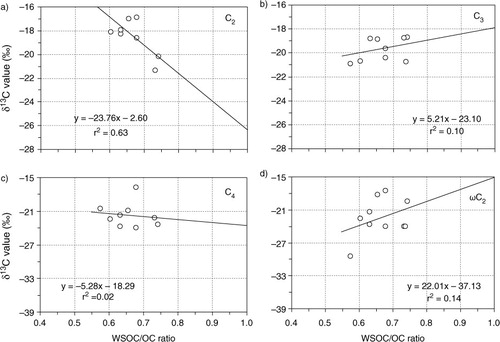
Fig. 3 Box plot of stable carbon isotope ratios of diacids, glyoxylic acid (ωC2) and glyoxal (Gly) in PM2.5 and PM10 aerosols from Morogoro, Tanzania, collected during the campaign. Each box shows the median (black line), the interquartile range (box) and the minimum and maximum values. Open circles show the outliers.
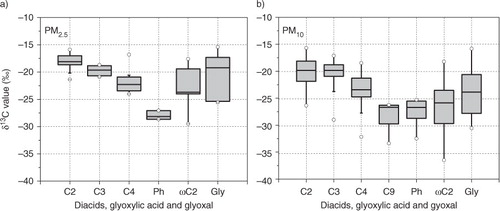
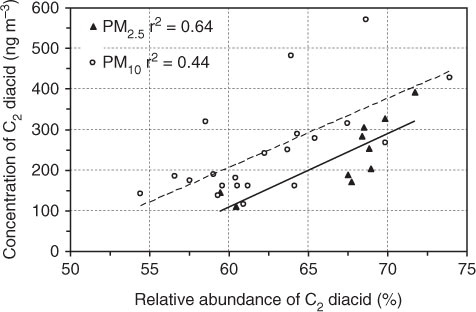
Fig. 4 Relation between concentrations of oxalic (C2) acid and its relative abundance to total diacids in PM2.5 and PM10 during 2011 sampling period in Morogoro, Tanzania. Data from Mkoma and Kawamura (2013).