Figures & data
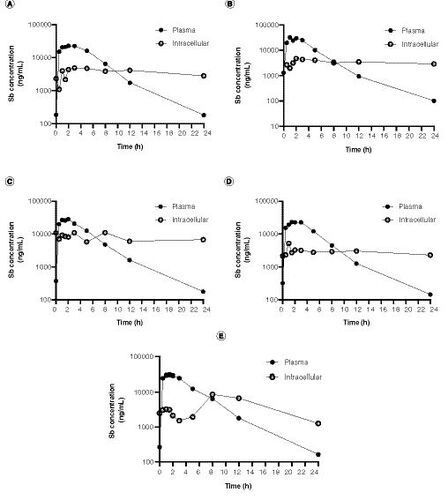
Table 1. Clinical characteristics of cutaneous leishmaniasis patients.
Table 2. Agilent 7700x operating parameters.
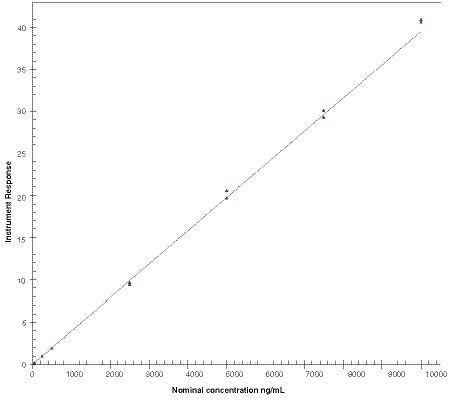
Table 3. Calibration curve regression parameters for antimony.
Table 4. Back-calculated calibration standards for antimony in human plasma.
Table 5. Accuracy and precision of antimony in human plasma.
Table 6. Accuracy and precision of antimony in human peripheral blood mononuclear cells.
Table 7. Validated methods for antimony quantification in human samples.
![Figure 1. Proposed meglumine antimoniate chemical structure.Proposed structural formula for meglumine antimoniate (364 Da) in aqueous solution.Adapted with permission from [Citation23].](/cms/asset/fe4e90dd-d428-4ce3-8cf6-08303cd8786e/ibio_a_12352723_f0001.jpg)