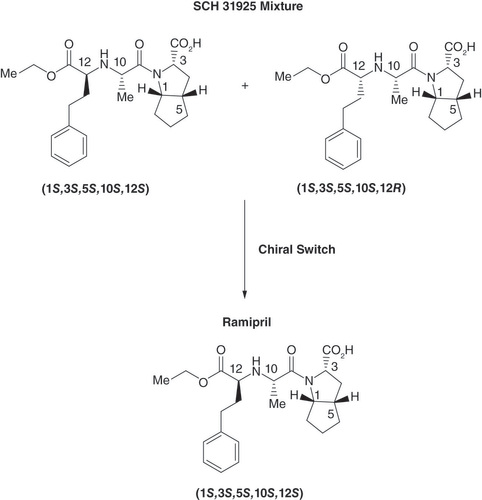
Figures & data
The IUPAC names of quinine and quinidine are (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol and (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol, respectively. Note that the numbering of atoms in the IUPAC names differs from the conventional numbering of the atoms of quinine and quinidine.
![Figure 1. Chemical structures of Cinchona alkaloids.The IUPAC names of quinine and quinidine are (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol and (S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol, respectively. Note that the numbering of atoms in the IUPAC names differs from the conventional numbering of the atoms of quinine and quinidine.](/cms/asset/89ae3494-15be-46af-bb1a-c83423e2957d/ifdd_a_12367014_f0004.jpg)