Figures & data
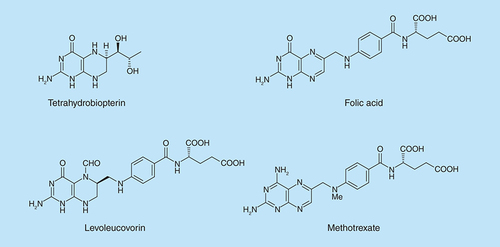
Reagents and conditions: (A) Na, ethanol, reflux. 2 h; (B) methyl iodide, ethanol, reflux 1.5 h; (C) NaNO2 aqueous acetic acid, 0°C, 2 h then 4°C, 16 h; (D) 1-methylpiperazine ethanol, reflux 0.75 h then add water and reflux 0.75 h; (E) sodium dithionite then aqueous 40% glyoxal (5a) or butane-2,3-dione (5b, or benzil [5c]), reflux 8 h, 18 h and 24 h, respectively.
![Figure 2. Synthesis of pteridines (5).Reagents and conditions: (A) Na, ethanol, reflux. 2 h; (B) methyl iodide, ethanol, reflux 1.5 h; (C) NaNO2 aqueous acetic acid, 0°C, 2 h then 4°C, 16 h; (D) 1-methylpiperazine ethanol, reflux 0.75 h then add water and reflux 0.75 h; (E) sodium dithionite then aqueous 40% glyoxal (5a) or butane-2,3-dione (5b, or benzil [5c]), reflux 8 h, 18 h and 24 h, respectively.](/cms/asset/3ecd2469-f47d-49c7-96af-e3e08c0ccbfe/ifmc_a_12363343_f0002.jpg)
Reagents and conditions: (A) 1-methylpiperazine, reflux, 18 h; (B) NaNO2 aqueous acetic acid, 0°C, 3 h; (C) sodium dithionite then aqueous 40% glyoxal, reflux 7 h; (D) NaHB(OAc)3 (9 eq for 10a and 1 eq for 10b), acetic acid 48 h, 20°C.
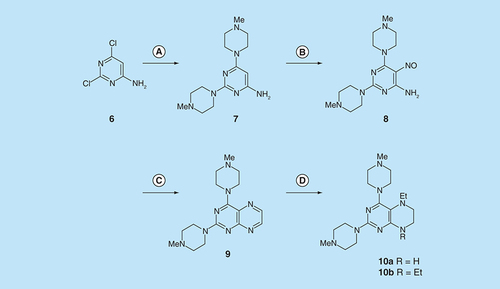
Reagents and conditions: (A) 3-hydroxypiperazine, reflux 5 h; (B) NaNO2 aqueous acetic acid 0°C, 3 h; (C) sodium dithionite then aqueous 40% glyoxal, reflux 6 h.
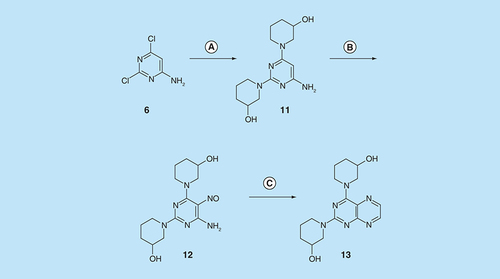
Reagents and conditions: (A) R1CH2NH2 diglyme, reflux, 5 h; (B) NaNO2 aqueous acetic acid, 0°C, 2 h then 4°C, 16 h; (C) 1-methylpiperazine or 4-methyl-1,4-diazepane, ethanol, reflux, 2 h then add water and reflux 1 h; (D) sodium dithionite then aqueous 40% glyoxal or butane-2.3-dione, reflux.
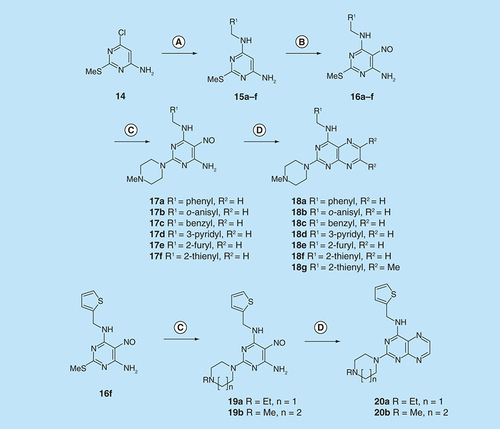
Reagents and conditions: (A) H2, 10% Pd on C, ethanol; then NaNO2, glacial acetic acid, 90°C, 2 h; (B) sodium thiosulfate pentahydrate, aq. 20% acetic acid 90°C, 1.5 h; (C) lead tetraacetate, acetic acid, 20°C, 4 h.
Energy minimizations were carried out using the AMBER99SB-ILDN force field [Citation41] with GROMACS as the molecular simulation toolkit [Citation42]. AutoDock Vina (1.1.2) [Citation41] was used for docking. Iron is rendered as a brown sphere. Prepared using PyMOL, this figure represents the preferred pose according to scoring function.
![Figure 7. Docking pose of pteridine 18d (depicted in turquoise) bound to soybean lipoxygenase (LOX-1) derived by modification of PDB code: 3PZW.Energy minimizations were carried out using the AMBER99SB-ILDN force field [Citation41] with GROMACS as the molecular simulation toolkit [Citation42]. AutoDock Vina (1.1.2) [Citation41] was used for docking. Iron is rendered as a brown sphere. Prepared using PyMOL, this figure represents the preferred pose according to scoring function.](/cms/asset/c6fa3bc8-3f7b-41da-be5d-2afa6a09e389/ifmc_a_12363343_f0007.jpg)