Figures & data
The figure shows as, by an unsupervised hierarchical clustering (similarity measure: Pearson centered; linkage rule: average), we were able to separate control (ten fresh-frozen motor cortex samples from individuals who had died from a non-neurological disease) from SALS patients (31 fresh-frozen motor cortex samples) on the basis of similarity in their expression patterns in motor cortex (considering the 9646 most informative genes, with an SD >1.5). In addition, this clustering allowed us to segregate sproadic amyotrophic lateral sclerosis (SALS) patients in two greatly divergent groups (SALS1 and SALS2). In the 2D presentation, each row represents a single gene and each column a motor cortex from control or SALS patients. Highly expressed genes are shown in red, downregulated genes in blue, no change in white. In the dendrograms shown (left and top), the length and the subdivision of the branches display the relatedness of the expression of the genes (left) and the motor cortex (top). The disease state is marked as follows: controls patients are indicated by blue rectangles and SALS patients by red rectangles. In the cluster panel, blue rectangle refers to control patients, yellow to SALS1 and orange to SALS2 patients. For further details, the reader is referred to [Citation7].
![Figure 1. Unsupervised hierarchical clustering of control and sporadic amyotrophic lateral sclerosis patients.The figure shows as, by an unsupervised hierarchical clustering (similarity measure: Pearson centered; linkage rule: average), we were able to separate control (ten fresh-frozen motor cortex samples from individuals who had died from a non-neurological disease) from SALS patients (31 fresh-frozen motor cortex samples) on the basis of similarity in their expression patterns in motor cortex (considering the 9646 most informative genes, with an SD >1.5). In addition, this clustering allowed us to segregate sproadic amyotrophic lateral sclerosis (SALS) patients in two greatly divergent groups (SALS1 and SALS2). In the 2D presentation, each row represents a single gene and each column a motor cortex from control or SALS patients. Highly expressed genes are shown in red, downregulated genes in blue, no change in white. In the dendrograms shown (left and top), the length and the subdivision of the branches display the relatedness of the expression of the genes (left) and the motor cortex (top). The disease state is marked as follows: controls patients are indicated by blue rectangles and SALS patients by red rectangles. In the cluster panel, blue rectangle refers to control patients, yellow to SALS1 and orange to SALS2 patients. For further details, the reader is referred to [Citation7].](/cms/asset/015996e5-60e0-42e2-ac3f-b05ebc1b1109/ifmc_a_12363292_f0001.jpg)
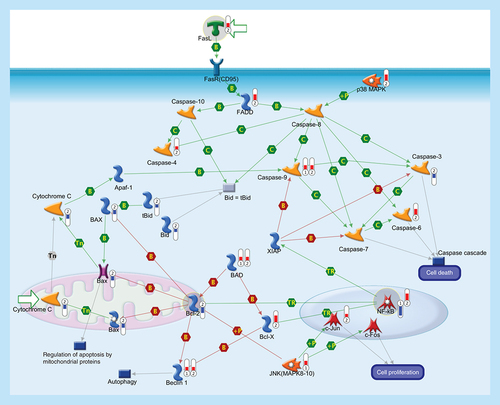
Apoptosis is a programmed cells death regulated through a variety of different pathways which interact and eventually lead to controlled cell death. The activation of the apoptotic response occurs through either the intrinsic or the extrinsic pathway, depending on the origin of the death stimuli. The extrinsic apoptosis is triggered by the interaction of an extracellular death ligand (e.g., FasL) to its cell-surface death receptor FasR (CD95), triggering movement of cytosolic adaptor proteins (e.g., FADD) to the intracellular portion of the receptor and, together with protein kinase p38 MAPK, transducing cell-death signals to downstream effector caspases that are responsible for the biochemical aspects of apoptosis. The intrinsic pathway is associated with mitochondrial membrane permeabilization and release of cytochrome c into the cytoplasm. The release of cytochrome c into the cytosol, in response to mitochondrial injury, triggers the recruitment of procaspase-9 to form a mitochondrial apoptosome and subsequent caspase activation. Sequential activation of caspases by the intrinsic or extrinsic signaling pathways plays a central role in the execution-phase of apoptosis. Bcl-2 family comprises a group of structurally related proteins (Bcl-2, Bcl-X, Bax, Bad, Bid) that play a fundamental role in the regulation of the intrinsic pathway by controlling mitochondrial membrane permeability and the release of the pro-apoptotic factor, cytochrome c. Caspase cascade is also involved in the autophagy, a catabolic process involved in cell death and in cell protective mechanism. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Activation of transcription factor NF-κB, a key mediator of genes involved in the control of the cellular proliferation and apoptosis, can promote neuronal survival by inducing the expression of genes encoding antiapoptotic proteins (e.g., XIAP). Activated JNK (MAPK8–10) induces cell apoptotic process (e.g., by phosphorylating mitochondrial proteins, such as the antiapoptotic proteins Bcl-XL and Bcl-2), or can mediate cell proliferation, regulation of apoptotic process and inflammatory response (mainly by phosphorylating transcription factors of the AP-1 family such as c-Jun, and c-Fos). Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometers have red color and indicate upregulated signals in SALS patients, downward (blue) ones indicate downregulated signals. Pathway objects and links are described separately in the Supplementary Figure 1.
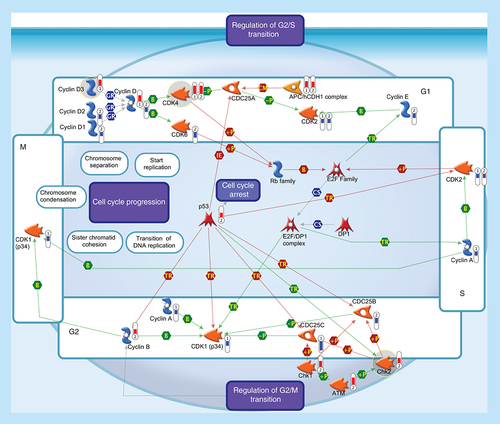
All somatic eukaryotic cells proceed through four phases in cell cycle: G1 (gap phase 1) in which the cell prepares for the upcoming events of S-phase; S (synthesis phase), in which DNA is replicated; G2 (gap phase 2) in which the cell prepares for the upcoming events of M-phase; and M (mitosis), in which chromosomes are separated over two new nuclei. Regulation of the cell cycle involves several protein kinases (such as the Checkpoint homologs Chk1, Chk2) that, activated by ATM, inhibit CDC25 phosphatase, preventing entry into mitosis and stabilize the tumor suppressor protein p53, leading to cell cycle arrest in G1. Progression through the cell-division cycle is driven by activation and inactivation of CDKs, which are controlled by periodic synthesis and degradation of cyclins and require association with a cyclin subunit for their activation. Cyclin/CDK complexes trigger the transition to next stage of the cell cycle: Cyclin D/CDK4 (or CDK6) for G1 progression, Cyclin E/CDK2 for the G1-S transition, Cyclin A/CDK2 for S-phase progression and Cyclin A/CDK1 and Cyclin B/CDK1 for entry into M-phase. Cyclin D, the first cyclin produced in the cell cycle in response to extracellular signals (e.g., growth factors), binds to existing CDK4, forming the active cyclin D-CDK4 complex that, in turn, phosphorylates the retinoblastoma family of tumor suppressor proteins (Rb family) liberating and activating the E2F transcription factors. These factors are associated with DP1 and their activation results in transcription of various genes like Cyclin E, Cyclin A, CDK1 and products that are necessary for the replication of DNA and beginning of the S phase. The activity of a Cyclin/CDK complex can be inhibited by phosphorylation at a pair of amino acids in the roof of the active site, while dephosphorylation of these sites by a phosphatase known as CDC25 (CDC25A, CDC25B and CDC25C) increases CDK activity. Events controlling cell division are governed by the degradation of different regulatory proteins by the ubiquitin-dependent pathway. Phosphorylated CDC25A may be exposed to ubiquitination by APC, one of ubiquitin ligases, which is known to be essential in maintaining normal cell cycle. Thermometers represent expression ratio in motor neuron among following conditions: 1 (SALS1/control) and 2 (SALS2/control). Upward thermometrs have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.
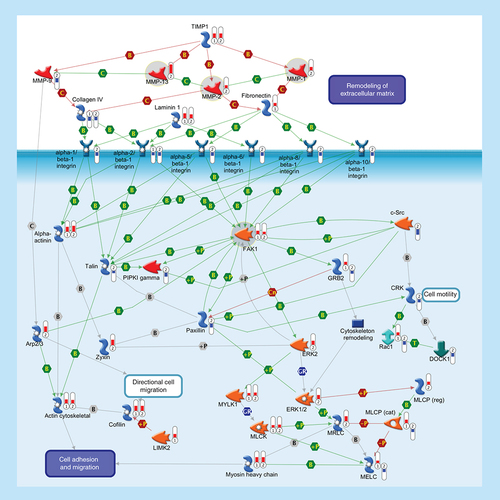
ECM remodeling is involved in normal physiological processes, such as cell motility and adhesion. MMPs are a family of proteolytic enzymes that degrade various components of the ECM in these processes. Endogenous TIMPs, such as TIMP1, reduce excessive proteolytic ECM degradation by MMPs. Integrins are heterodimeric cell surface adhesion receptors formed by two noncovalently associated subunits, alpha and beta. Most integrins recognize several ECM proteins, such as Laminin 1, Fibronectin and Collagen IV, whereas alpha-5/beta-1 integrin recognizes only Fibronectin. The ECM, integrins and the cell cytoskeleton interact at sites called focal contacts. The integrin-binding proteins Paxillin and Talin recruit FAK1 to focal contacts. Activated Talin binds to PI(4,5)P2 producing enzyme PIPKI gamma and activates it. PIPKI gamma also can be stimulated by tyrosine-protein kinase c-Src and FAK1 phosphorylation. Integrin clustering promotes FAK1 autophosphorylation, thereby creating a binding site for c-Src. Phosphorylation of FAK1 by c-Src maximizes catalytic activity of FAK1 and creates a binding site for GRB2, thereby leading to the activation of ERK1/2. ERK2 phosphorylates FAK1 and decreases Paxillin binding to FAK1. Within focal contacts, FAK1-c-Src-mediated phosphorylation of Paxillin promotes ERK2 binding. ERK2-mediated phosphorylation of Paxillin can facilitate FAK1 binding to Paxillin and enhance FAK1 activation. ERK2-mediated phosphorylation and activation of MYLK1 contributes to cell-matrix adhesion dynamics. Alpha-actinin is a cytoskeletal protein that crosslinks Actin in actomyosin stress fibers and tethers them to focal contacts. Phosphorylation of Alpha-actinin by FAK1 reduces the crosslinking of stress fibers and prevents maturation of the focal contacts. The Arp2/3 nucleates new Actin filaments from the sides of pre-existing filaments. Activation of LIMK2 leads to phosphorylation and inactivation of actin depolymerizing factor Cofilin, resulting in net increase in the cellular filamentous Actin cytoskeletal. Zyxin is an Alpha-actinin and stress-fiber-binding protein found in mature contacts. Inactivation of the MLCP attenuates phosphorylation of the MELC, MLCK and MRLC, inhibiting the formation of actomyosin stress fibers. CRK facilitates activation of Rac1 by DOCK1. Rac1 leads to activation of MRLC and the Arp2/3 complex. Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometrs have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.
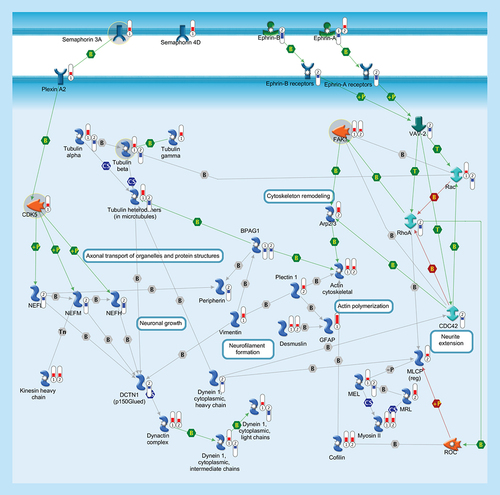
Cytoskeleton consists of three distinct, yet interconnected filament systems: microfilaments (actin filaments), intermediate filaments and microtubules. Microfilaments are the thinnest filaments of the cytoskeleton and are composed of linear polymers of actin subunits. They act as tracks for the movement of myosin molecules and are controlled by the Rho family of small GTP-binding proteins such as Rho, Rac and Cdc42. The activation of RhoA downstream effector Rho-associated kinase ROCK phosphorylates and inactivates the actin-associated protein cofilin, leading to reorganization of the actin cytoskeleton. Intermediate filaments (vimentin, desmusin, gfap, peripherin, NEFL, NEFM, NEFH) organize the internal tridimensional structure of the cell, anchoring organelles and serving as structural components of the nuclear lamina and sarcomeres. Intermediate filament binding protein Plectin 1 interacts with the Actin cytoskeleton to promote the formation of actin-rich structures that are important for cell motility. Neurofilaments are important protein cargoes for actin-associated motors, such as myosin, and microtubule-associated motor, such as kinesin in a complex with Dynactin. Microtubules are polymers of alpha and beta tubulin and, in association to other proteins such as Dynein and Dynactin, are important for axonal transport. Semaphorins act as axonal guidance factors. Semaphorin 3A binds to Plexin A2 and induces repulsive responses. Ephrins and their receptor protein-tyrosine kinases activate several factors (e.g., VAV-2) and regulate a variety of biological processes including the guidance of axon growth. Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometrs have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.
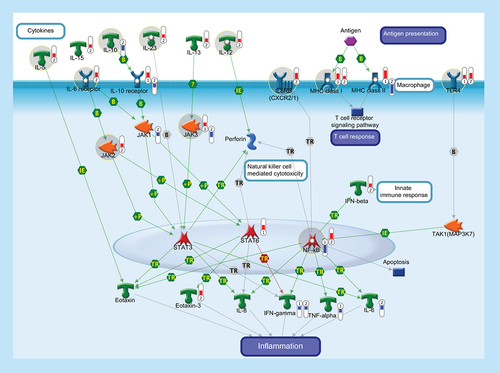
The function of the immune system depends on a large part on ILs, a group of cytokines involved in either immunologic or inflammatory response. ILs are mainly secreted by mononuclear cells that induce the growth and differentiation of lymphocytes and pluripotential stem cells. IL-5 is produced by Th2 cells and mast cells after activation by mitogens or antigens and is capable to induce the survival, growth and differentiation of eosinophils and B-cells. IL-10 is a pleiotropic cytokine with important immunoregulatory functions and potent anti-inflammatory properties. Binding of IL-10 to the extracellular domain of IL-10 receptor, together with the activation of IL6 receptor, activates phosphorylation of the receptor-associated JAK and STAT. Activation of JAK/STAT cascades is involved in the transcriptional regulation of several genes that regulate chemotaxis, inflammation, cell proliferation and migration (such as IL-8, Eotaxin, IFN-γ, TNF-α, Perforin and IL-6). IL-12 is a key immunoregulatory cytokine that coordinates innate and adaptive immune response. IL-13 plays a critical role in the regulation of immune responses and is also implicated in pathological conditions, such as asthma and allergy. IL-15 is a pleiotropic cytokine involved in several cellular process, such as proliferation, differentiation, immune response and cell survival. IL-23 plays an important role in differentiation and maintaining the Th17 cell population, a novel T-cell subset involved in antimicrobial immune response and establishment of many autoimmune diseases. IL-8 or CXCL8 is a chemokine produced by macrophages and other cell types that, through its binding to IL8 receptors (also known as CXCR1 and CXCR2), mediates several cellular functions, including neutrophil migration to sites of inflammation. IL-8 promotes NF-κB binding to DNA and transcriptionally regulates the expression of several factors involved in immune and inflammatory response. MHC class I molecules are specialized for presentation of endogenously synthesized proteins, bind ER chaperone calreticulin and then antigenic peptides. MHC class II molecules are found on antigen-presenting cells and present antigen derived from extracellular proteins (not cytosolic as in class I). TLR4, FCRL1 and FCRL2 are monocyte/macrophage-specific genes. Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometrs have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.
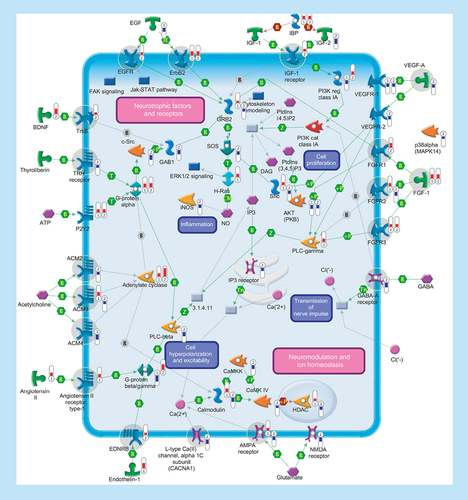
Several genes encoding ligands and receptors involved in signal transduction were differentially expressed in SALS patients. Signaling pathways are separated into two subsections (‘Neurotrophic factors and receptors’ and ‘Neuromodulation and ion homeostasis’), including primary (such as aAdenylate cyclase, phospholipase C or tyrosine kinase receptors) and secondary (such as Ca2+ release, PKC or protein kinase cascades) effectors that are regulated at multiple levels with different mechanisms (such as binding or phosphorylation). Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometers have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.
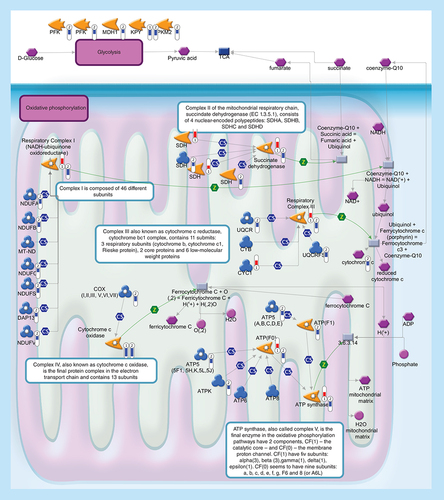
Glycolysis (upper level) converts glucose into pyruvate. During oxidative phosphorylation (lower level) electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions, carried out by five main protein complexes, release energy that is used to form ATP. The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called chemiosmosis. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. Protons flow back across the membrane and down this gradient through ATP synthase, an enzyme that generates ATP from ADP, in a phosphorylation reaction. Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometrs have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.
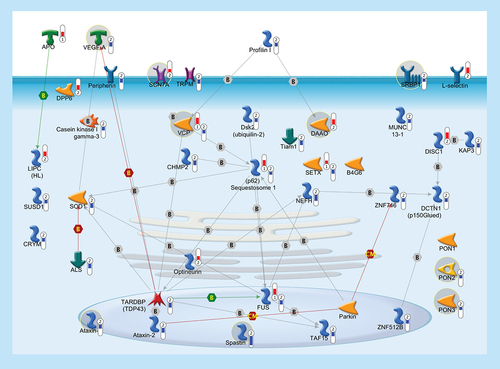
In order to highlight a possible common pathogenic mechanism between familiar and sporadic ALS, we built up a pathway with 41 genes, some of which (37/41), besides being previously linked to FALS, were significantly deregulated in the cortex of SALS patients. Thermometers represent expression ratio in motor neuron among following conditions: (SALS1/control) and (SALS2/control). Upward thermometrs have red color and indicate expression ratios less than 1 while downward thermometers have blue color and indicate expression ratio less than 1. Pathway objects and links are described separately in the Supplementary Figure 1.