Figures & data
Figure 1. Organization of the androgen receptor gene and protein.
The Ar gene is located on the long arm of the X-chromosome. The coding sequence consists of eight exons which give rise to the four structural and function domains of the receptor protein (exons and protein domains appear in the same colors). The location of the polymorphic amino acid repeats polyglutamine (Q21) and -glycine (G23) are indicated.
DBD: DNA binding domain; HR: Hinge region; LBD: Ligand binding domain; NTD: N-terminal domain.
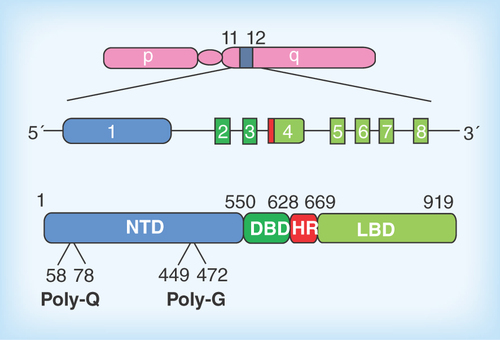
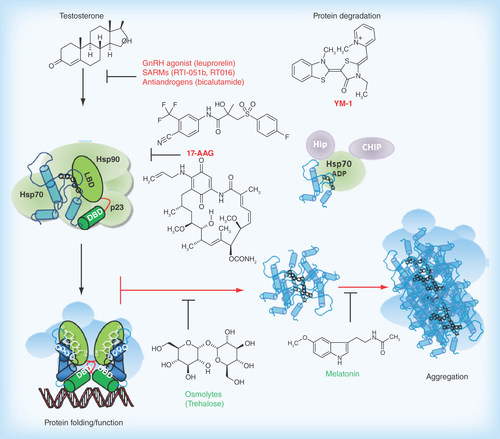
Figure 2. Pathophysiological action of the AR protein and potential sites for therapeutic intervention in spinal-bulbar muscular atrophy.
Left Panel: in the absence of hormone the AR is located in the cytoplasm in a multiprotein complex) including molecular chaperones (hsp90, hsp70; green shapes). Upon binding hormone, the receptor translocates to the nucleus and binds as a dimer to specific DNA sequences and interacts with co-regulatory proteins and the transcription machinery (blue shapes). Right Panel; The AR-NTD is structurally flexible and the expanded AR poly-Q tract allows transition into a distinct conformation that may cause toxicity as a monomer or it may self-associate to form toxic oligomers, which could assemble into larger aggregates leading to intracellular inclusions. Formation of aggregates may also involve proteolysis and the generation of poly-Q containing N- terminal domain polypeptides. The principal toxic effects of the aberrantly folded protein may include alterations in transcription (through sequestering of co-regulatory proteins; blue shapes), metabolism or impairment of the proteasome or stress response pathways. Allosteric activators of Hsp70, including Hip and the small molecule YM-1 together with chaperone-dependent ubiquitin ligases, such as CHIP (green and purple shapes), increase substrate binding affinity, facilitate client protein ubiquitination and promote poly-Q AR clearance by the proteasome. Hormone binding and nuclear translocation can be prevented using GnRH agonists or possibly antiandrogens, while inhibiting Hsp90 (17-AAG) can block hormone binding and lead to degradation of the AR protein. Chemical chaperones, known as osmolytes may allow misfolded AR back into a native conformation, and thereby restore proper folding and functions of the receptor, while melatonin has been shown to reverse aggregate formation of the mutant receptor. See text for details and relevant references.