Figures & data
Figure 1. Myocardial injury triggers cardiogenesis gene expression.
(A) Schematic diagram of the analysis of cardiogenesis gene expression after myocardial injury. The hearts were harvested from the mice before surgery and 1, 3, 5, 7, 9, 11, 14 and 21 days following MI. (B) Gene expression of the hearts before and after MI. In the external and internal parts of the hearts, expression of the cardiogenesis genes GATA4 and Nkx2.5 increased after MI. GATA4 expression peaked on day 11, and Nkx2.5 expression peaked on day 21 post MI (n = 4 to 5 in each group).
MI: Myocardial injury.
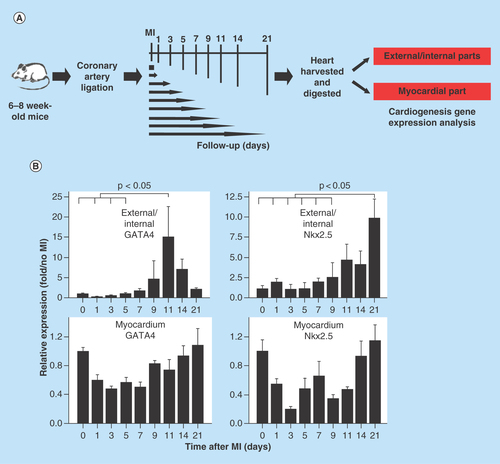
Figure 2. Cardiomyogenic potential of Nkx2.5 enhancer-expressing cells.
(A) Nkx2.5 enh-eGFPpositive cells sorted from the heart of postnatal Nkx2.5 enh-eGFP mice were cultured to test the cardiomyogenic potential of postnatal Nkx2.5 enhancer-expressing cells. Initially post sorting (2 days later), Nkx2.5 enh-eGFPpositive cells stained negative for cTnI. After culture for 10 days, isolated Nkx2.5 enh-eGFPpositive cells differentiated into cTnI+ cardiomyocytes with striation. (B) Immunostaining of activated Nkx2.5 enh-eGFPpositive cells with precardiac mesoderm marker (GATA4) and cardiac precursor marker (Nkx2.5) from the hearts of Nkx2.5 enh-eGFP mice 1 week following MI. The eGFP+ cells stained positive for GATA4 and Nkx2.5.
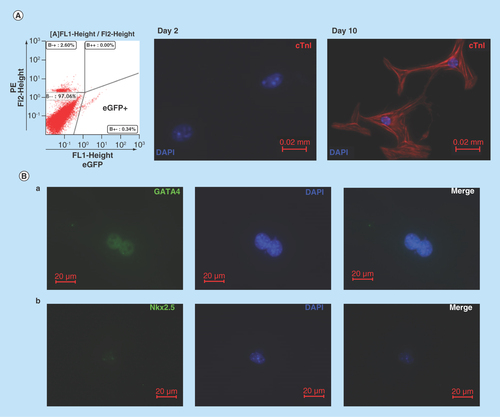
Figure 3. In vivo evidence that Nkx2.5 enhancer-expressing cells differentiate into cardiomyocytes.
(A) Lineage tracing of Nkx2.5 enhancer-expressing cells after MI. Representative LacZ-stained heart sections from the inducible Nkx2.5 enh-Cre/R26R-LacZ mice with tet-off system. (a) Unoperated group, treated with doxycycline from conception to sacrifice. (b) 6 weeks post MI and treated with doxycycline from conception to MI. Note some cells in the subepicardium and myocardium stained positive for LacZ. Arrows indicate infarct area. (c)-(d) Higher magnification of (b). (B) Immunostaining of representative heart sections from the inducible Nkx2.5 enh-Cre/R26R-LacZ mice, 6 weeks post MI and treated with doxycycline from conception to MI. The descendent cells of activated Nkx2.5 enhancer-expressing cells (red fluorescence; arrows, labeled by anti-β-galactosidase antibody, BGal) expressed cardiomyocyte marker cardiac troponin T (cTnT) (green fluorescence; arrow heads). Cell nuclei visualized with DAPI staining.
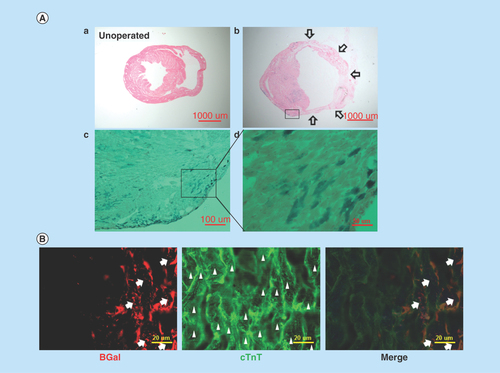
Figure 4. Expansion of Nkx2.5 enh-eGFPpositive cells after experimental MI.
(A) Schematic diagram of the analysis of Nkx2.5 enh-eGFPpositive cells after coronary ligation. (B) Flow cytometry of the percentage of Nkx2.5 enh-eGFPpositive cells before and 2 weeks following MI. The baseline cell percentage of Nkx2.5 enh-eGFPpositive cells was low, but increased markedly by 2 weeks following MI. (C) Quantification of the percentage of Nkx2.5 enh-eGFPpositive cells in the mice before and 1, 3, 5, 7, 11, 14 and 21 days following MI. The Nkx2.5 enh-eGFPpositive cells increased after surgery and were activated up to 14-fold 2 weeks following MI (n = 4 to 5 in each group).
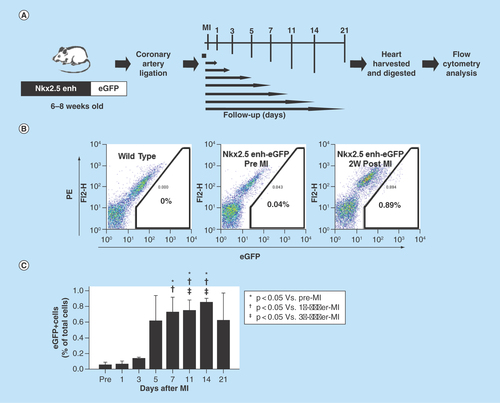
Figure 5. Lineage tracing of the postnatal Nkx2.5 cardiac progenitor cells.
(A) The strategy used to analyze the developmental origin of activated postnatal Nkx2.5 cardiac progenitor cells by excision PCR on sorted Nkx2.5 enh-eGFP+ cells from the hearts of different lineage-Cre/Nkx2.5 enh-eGFP/R26R-LacZ mice. (B) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of endothelial cell lineage, Tie2-Cre/Nkx2.5-eGFP/R26R-LacZ mice. The positive control was the DNA from the unsorted cells of triple transgenic mouse heart. The negative excision bands of the sorted eGFP+ cells confirmed that the activated Nkx2.5 enh-eGFPpositive cells following MI did not originate from the endothelial cells. (C) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of cardiac neural crest cell lineage, Pax3-Cre/Nkx2.5-eGFP/R26R-LacZ mice. The result showed that the activated Nkx2.5 enh-eGFPpositive cells did not derive from the cardiac neural crest cells. (D) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of cardiomyocyte lineage, αMHC-MerCreMer/Nkx2.5 enh-eGFP/R26R-LacZ mice. Pre-existing cardiomyocytes were labeled with 4-OH tamoxifen prior to MI. The results showed that the activated Nkx2.5 enh-eGFPpositive cells following MI did not originate from the pre-existing cardiomyocytes.
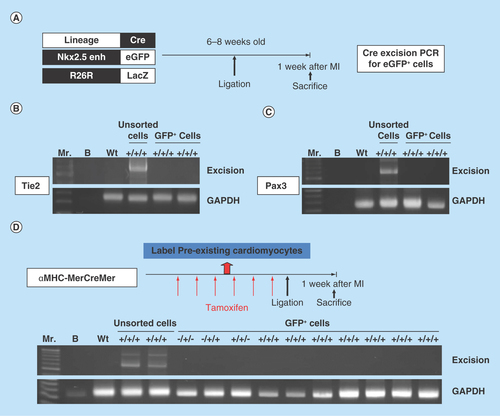
Figure 6. Postnatal cardiac progenitor cells originate from the embryonic epicardial cells.
(A) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of epicardial cell lineage, GATA5-Cre/Nkx2.5 enh-eGFP/R26R-LacZ mice. The positive excision bands (arrows) of the sorted Nkx2.5 enh-eGFPpositive cells suggested the epicardial origin of postnatal Nkx2.5 cardiac progenitor cells. (B) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of adult epicardial lineage, Wt1CreERT2/Nkx2.5 enh-eGFP/R26R-LacZ mice. The peri-MI Wt1 cells were labeled with 4-OH tamoxifen before and after MI. The result showed that the activated Nkx2.5 enh-eGFPpositive cells did not arise from the peri-MI Wt1 epicardial cells. (C) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of perinatal epicardial lineage, Wt1CreERT2/Nkx2.5 enh-eGFP/R26R-LacZ mice, with labeled perinatal Wt1 cells via 4-OH tamoxifen injection on ED 18.5. The results showed that the activated Nkx2.5 enh-eGFPpositive cells did not derive from the perinatal Wt1 epicardial cells. (D) Excision PCR of the activated Nkx2.5 enh-eGFPpositive cells from the hearts of embryonic epicardial lineage, Wt1CreERT2/Nkx2.5 enh-eGFP/R26R-LacZ mice, labeled the embryonic Wt1 cells with 4-OH tamoxifen injection on ED 10.5. The positive excision band (arrow) of the sorted Nkx2.5 enh-eGFPpositive cells suggestive of the embryonic epicardial origin of postnatal Nkx2.5 cardiac progenitor cells.
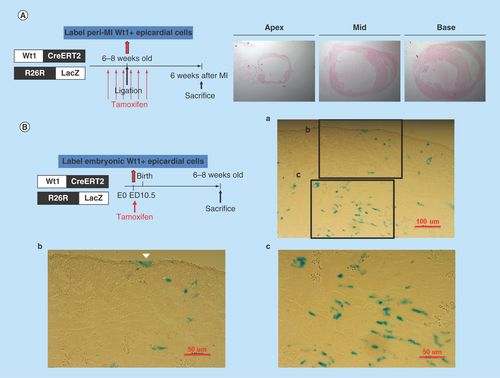
Figure 7. LacZ staining of heart sections from the Wt1CreERT2/R26R-LacZ mice.
(A) Peri-MI Wt1 cells were labeled with 4-OH tamoxifen for 1 week prior to MI and for 1 week after MI. No LacZ+ cells were noted, indicating that very few Wt1 epicardial cells existed in the adult heart, even after myocardial injury. (B) LacZ staining of the representative heart sections from the 6- to 8-week-old Wt1CreERT2/R26R-LacZ mice labeled the embryonic Wt1 cells with 4-OH tamoxifen injection on ED 10.5. Note the epicardial cells (arrow head) and other cardiac cells were labeled LacZ positive.