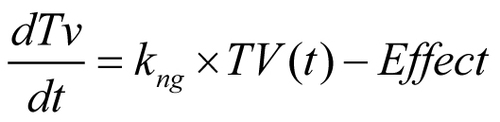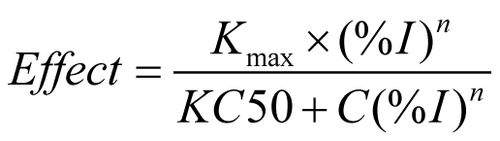Figures & data
Figure 1. Comparison of number of days to finalize budget and contract, days to Institutional Review Board approval, days to first subject enrollment and total start-up time between different study types.
Total study start-up time is defined as site receipt of the feasibility questionnaire to first subject enrollment. Bars represent standard error for each mean value.
IRB: Institutional Review Board.
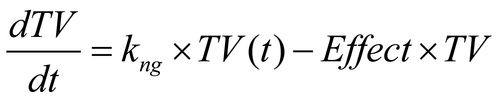
Table 1. Median and 75th percentile number of days to budget and contract finalization, Institutional Review Board approval and first subject enrollment for each study type.
Figure 2. Comparison of number of days to finalize budget and contract, days to Institutional Review Board approval, days to first subject enrollment and total study start-up time with the presence and absence of a Contract Research Organization working on the study.
Total study start-up is defined as date of site receipt of the feasibility questionnaire to first subject enrollment. Bars represent standard error for each mean value.
CRO: Contract Research Organization; IRB: Institutional Review Board.
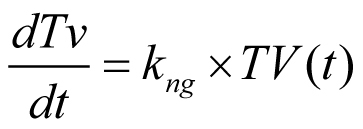
Figure 3. Comparison of number of days to finalize budget and contract, days to Institutional Review Board approval, days to first subject enrollment and total study start-up time based on project manager experience.
Total study start-up is defined as date of site receipt of the feasibility questionnaire to first subject enrollment. Forty-eight months was the approximate median experience time for all studies considered. Bars represent standard error for each mean value.
IRB: Institutional Review Board.
Figure 4. Comparison of number of days to finalize budget and contract, days to Institutional Review Board approval, days to first subject enrollment and total study start-up time based on number of ancillary services required by the study protocol.
Total study start-up is defined as date of site receipt of the feasibility questionnaire to first subject enrollment. Bars represent standard error for each mean value.
IRB: Institutional Review Board.
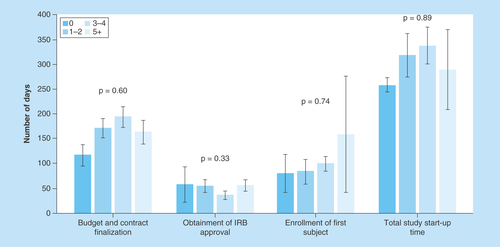
Figure 5. Comparison of number of days to Institutional Review Board approval with an Institutional Biosafety Committee approval requirement and without.
Bars represent standard error for each mean value.
IBC: Institutional Biosafety Committee; IRB: Institutional Review Board.
Figure 6. Comparison of number of days to Institutional Review Board approval using the local Institutional Review Board as compared to using a centralized Institutional Review Board.
Bars represent standard error for each mean value.
IRB: Institutional Review Board.
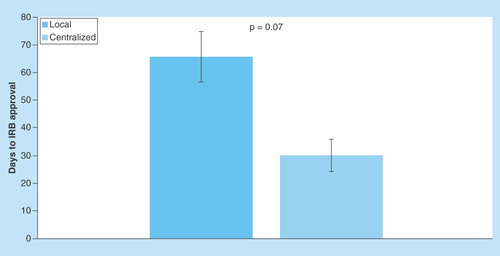
Figure 7. Comparison of number of days to finalize budget and contract, days to Institutional Review Board approval, days to first subject enrollment and total study start-up time for interventional (drug, device or biologic) studies as compared with observational studies.
Total study start-up is defined as date of site receipt of the feasibility questionnaire to first subject enrollment. Bars represent standard error for each mean value.
IRB: Institutional Review Board.
Figure 8. Comparison of the number of days taken to finalize the contract and budget for studies which obtained Institutional Review Board approval and those that were terminated before obtaining Institutional Review Board approval.
The number of days for contract and budget finalization for studies that eventually enrolled a subject are compared to those that were terminated prior to first subject enrollment. Bars represent standard error for each mean value.
IRB: Institutional Review Board.
Figure 9. The number of days taken to obtain Institutional Review Board approval for studies that eventually enrolled a subject are compared with those that were terminated before the first subject was enrolled.
Bars represent standard error for each mean value.
IRB: Institutional Review Board.
Figure 10. Cause and effect analysis of delays at the study start-up phase.
Causes specific to a study type are indicated by a symbol, with the legend in the bottom right corner.
†Biologic.
‡Device.
§Drug.
*Observational.
Table 2. Multivariate analysis of time to budget and contract approval, Institutional Review Board approval, and first patient enrollment as a function of Institutional Review Board type, project manager experience and use of a Contract Research Organization.
Table 3. Time to budget and contract finalization, Institutional Review Board approval, and first subject enrollment as a function of using a local Institutional Review Board, project manager experience <48 months and using a Contract Research Organization.
Table 4. Analysis of the features of studies which did not ultimately reach a negotiated contract and budget.
Table 5. Analysis of features of studies which did not ultimately obtain Institutional Review Board approval.
Table 6. Analysis of features of studies which did not ultimately enroll a first patient.